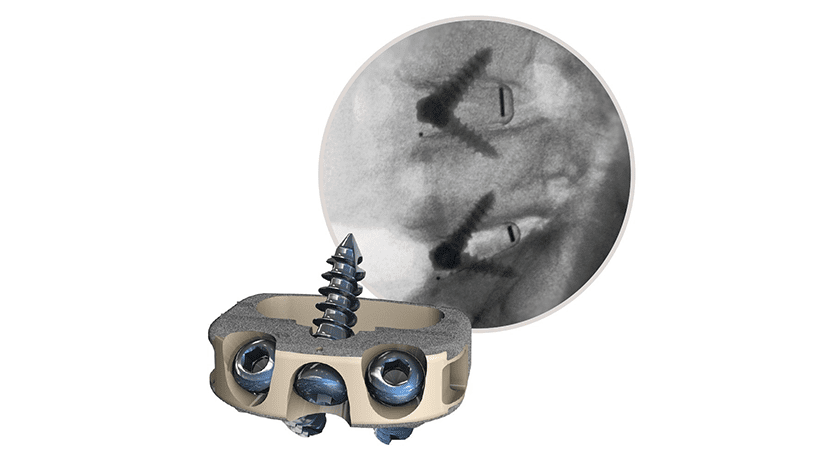






Results of retrospective study support the use of Centinel Spine’s STALIF® M portfolio of implants as standalone devices that produce good outcomes in patients treated for symptomatic disc degeneration while avoiding the use of posterior fixation and its complication risk and cost.
STALIF M stand-alone integrated cage-screw implants were utilized in a consecutive series of 58 patients with a mean follow-up time of 30.6 months (minimum 24-month follow-up). All available patients’ reported outcome scores demonstrated significant improvement. The results of this study corroborate that stand-alone ALIF is a viable procedure for the treatment of symptomatic disc degeneration in patients who have failed nonoperative care and who do not have specific indications for supplemental posterior instrumentation.
The STALIF M implant technology has been engineered based on the STALIF technology platform, which has a clinical history of over 30 years and over 75,000 devices implanted.
Source: Centinel Spine, LLC
Results of retrospective study support the use of Centinel Spine's STALIF® M portfolio of implants as standalone devices that produce good outcomes in patients treated for symptomatic disc degeneration while avoiding the use of posterior fixation and its complication risk and cost.
STALIF M stand-alone integrated cage-screw implants were...
Results of retrospective study support the use of Centinel Spine’s STALIF® M portfolio of implants as standalone devices that produce good outcomes in patients treated for symptomatic disc degeneration while avoiding the use of posterior fixation and its complication risk and cost.
STALIF M stand-alone integrated cage-screw implants were utilized in a consecutive series of 58 patients with a mean follow-up time of 30.6 months (minimum 24-month follow-up). All available patients’ reported outcome scores demonstrated significant improvement. The results of this study corroborate that stand-alone ALIF is a viable procedure for the treatment of symptomatic disc degeneration in patients who have failed nonoperative care and who do not have specific indications for supplemental posterior instrumentation.
The STALIF M implant technology has been engineered based on the STALIF technology platform, which has a clinical history of over 30 years and over 75,000 devices implanted.
Source: Centinel Spine, LLC


You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







