Copy to clipboard
Copy to clipboard 
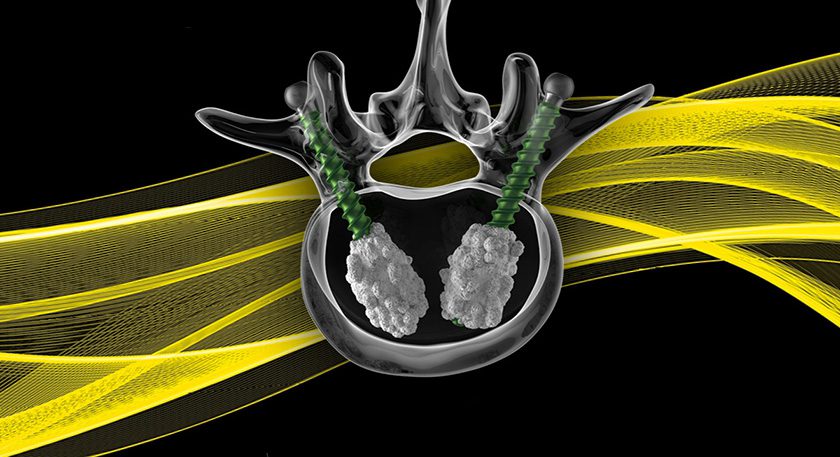
Results from ovine study of Bio2 Technologies’ Vitrium bioactive glass demonstrated the device’s capability to support interbody fusion.
The study compared the Vitrium device to a similar PEEK-based design, both used with autograft. Biomechanical tests evaluated subjects for:
- An effective, safe resorption/bone formation profile
- Stimulation of new bone formation to increase fusion rates
- Sufficient strength to bear/share physiologic loads
All subjects demonstrated a reduction in motion at 26 weeks, indicating fusion with both implants. In axial compressive load to failure testing, the Vitrium fusion exhibited strength in excess of the adjoining vertebral bodies, whereas the PEEK fusion failed at the fusion site.
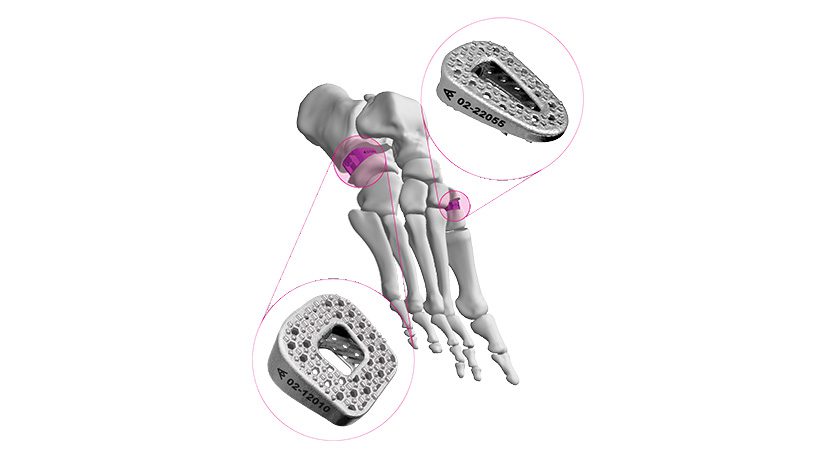
In late 2015, the company received FDA 510(k) clearance to market a Vitrium-based fusion system for interphalangeal fusion, fracture repair and osteotomies of toes, fingers and other small bones in the presence of appropriate immobilization.
Sources: Bio2Technologies, Inc.; bio2tech.com; ORTHOWORLD Inc.
Results from ovine study of Bio2 Technologies' Vitrium bioactive glass demonstrated the device's capability to support interbody fusion.
The study compared the Vitrium device to a similar PEEK-based design, both used with autograft. Biomechanical tests evaluated subjects for:
An effective, safe resorption/bone formation profile
...
Results from ovine study of Bio2 Technologies’ Vitrium bioactive glass demonstrated the device’s capability to support interbody fusion.
The study compared the Vitrium device to a similar PEEK-based design, both used with autograft. Biomechanical tests evaluated subjects for:
- An effective, safe resorption/bone formation profile
- Stimulation of new bone formation to increase fusion rates
- Sufficient strength to bear/share physiologic loads
All subjects demonstrated a reduction in motion at 26 weeks, indicating fusion with both implants. In axial compressive load to failure testing, the Vitrium fusion exhibited strength in excess of the adjoining vertebral bodies, whereas the PEEK fusion failed at the fusion site.
In late 2015, the company received FDA 510(k) clearance to market a Vitrium-based fusion system for interphalangeal fusion, fracture repair and osteotomies of toes, fingers and other small bones in the presence of appropriate immobilization.
Sources: Bio2Technologies, Inc.; bio2tech.com; ORTHOWORLD Inc.

You’ve reached your limit.
We’re glad you’re finding value in our content — and we’d love for you to keep going.
Subscribe now for unlimited access to orthopedic business intelligence.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.