
 Copy to clipboard
Copy to clipboard 
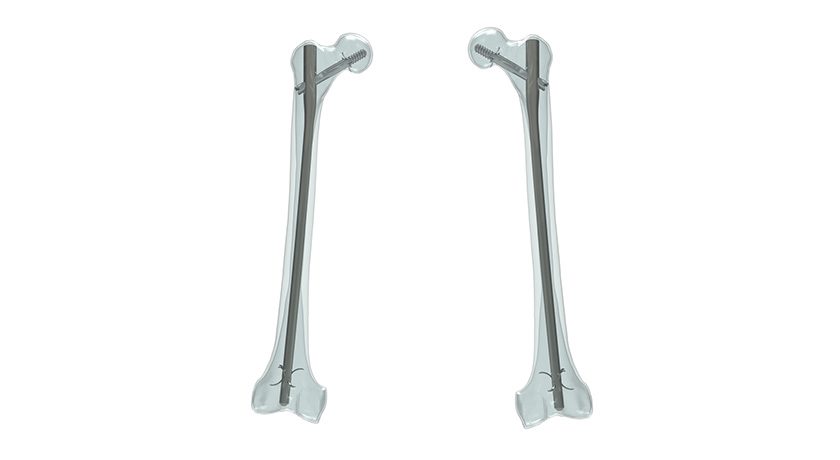
Orthopedic Designs North America (ODI) announced the successful completion of a study that evaluated the efficacy of its flagship product, the Talon DistalFix Proximal Femoral Nailing System. The study demonstrated that the product provides significant advantages for patients and surgeons alike.
The Talon DistalFix Proximal Femoral Nailing System is designed to treat proximal femoral fractures, and features Talon DistalFix technology, which is designed to eliminate the need for distal cortical screws and the associated freehand drilling. The system achieved similar fixation and union rates while enabling placement of a long protective construct, expeditious operative times, limited blood loss, limited radiation exposure, and an excellent return to prior functional level.
The study was conducted by a team of experienced orthopedic surgeons who evaluated the Talon DistalFix™ Proximal Femoral Nailing System in a clinical setting. The results of the study demonstrate the effectiveness of the Talon DistalFix technology and its ability to provide a streamlined surgical technique, reduced surgical time and improved patient outcomes.
“We are thrilled to see the positive results of this study,” said Charles Masek, CEO of ODI. “Our Talon DistalFix Proximal Femoral Nailing System is designed to provide the best possible outcomes for patients while also improving the surgical experience for the surgeon and the operating room staff. This study demonstrates the efficacy of our product and the significant advantages that it provides.”
Source: Orthopedic Designs North America, Inc.
Orthopedic Designs North America (ODI) announced the successful completion of a study that evaluated the efficacy of its flagship product, the Talon DistalFix Proximal Femoral Nailing System. The study demonstrated that the product provides significant advantages for patients and surgeons alike.
The Talon DistalFix Proximal Femoral Nailing...
Orthopedic Designs North America (ODI) announced the successful completion of a study that evaluated the efficacy of its flagship product, the Talon DistalFix Proximal Femoral Nailing System. The study demonstrated that the product provides significant advantages for patients and surgeons alike.
The Talon DistalFix Proximal Femoral Nailing System is designed to treat proximal femoral fractures, and features Talon DistalFix technology, which is designed to eliminate the need for distal cortical screws and the associated freehand drilling. The system achieved similar fixation and union rates while enabling placement of a long protective construct, expeditious operative times, limited blood loss, limited radiation exposure, and an excellent return to prior functional level.
The study was conducted by a team of experienced orthopedic surgeons who evaluated the Talon DistalFix™ Proximal Femoral Nailing System in a clinical setting. The results of the study demonstrate the effectiveness of the Talon DistalFix technology and its ability to provide a streamlined surgical technique, reduced surgical time and improved patient outcomes.
“We are thrilled to see the positive results of this study,” said Charles Masek, CEO of ODI. “Our Talon DistalFix Proximal Femoral Nailing System is designed to provide the best possible outcomes for patients while also improving the surgical experience for the surgeon and the operating room staff. This study demonstrates the efficacy of our product and the significant advantages that it provides.”
Source: Orthopedic Designs North America, Inc.

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







