
 Copy to clipboard
Copy to clipboard 
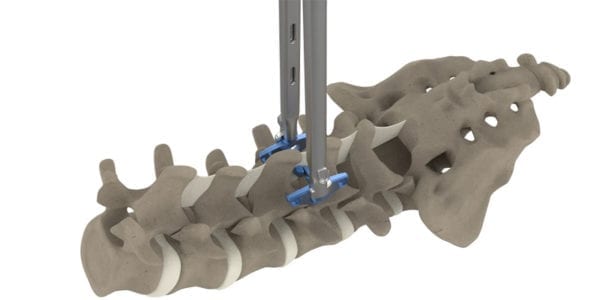
Clinical study results indicate the outcome of pain reduction after implanting the Inspan interspinous distraction decompression device. The study focused on outpatient interspinous lumbar fixation for degenerative intervertebral disc disorders with spinal stenosis using Inspan interspinous fixation. Unlike extension block designs, the Inspan device fixates the spine to allow immediate stability, distraction, decompression and fusion.
This retrospective study reports the clinical outcome of pain reduction after implanting the Inspan interspinous distraction decompression device. The study was conducted across 13 patients with a 68-year median age, all with chronic pain, and a 19-month median follow-up time. The procedures were performed on patients diagnosed with degenerative intervertebral disc disorders and underlying spinal stenosis. There were no complications, no revisions or any implant failures. In addition, post-op pain measured by the Numeric Pain Rating Scale showed statistically significant pain reduction for back and leg pain.
Dr. Pandey, President, and COO of Inspan, said, “Inspan has a long clinical track record since 2010, and we have continuously incorporated feedback from our doctors to improve the device, instruments, and technique. We will continue our commitment to innovation and listen to our doctors.”
Source: Inspan
Clinical study results indicate the outcome of pain reduction after implanting the Inspan interspinous distraction decompression device. The study focused on outpatient interspinous lumbar fixation for degenerative intervertebral disc disorders with spinal stenosis using Inspan interspinous fixation. Unlike extension block designs, the Inspan...
Clinical study results indicate the outcome of pain reduction after implanting the Inspan interspinous distraction decompression device. The study focused on outpatient interspinous lumbar fixation for degenerative intervertebral disc disorders with spinal stenosis using Inspan interspinous fixation. Unlike extension block designs, the Inspan device fixates the spine to allow immediate stability, distraction, decompression and fusion.
This retrospective study reports the clinical outcome of pain reduction after implanting the Inspan interspinous distraction decompression device. The study was conducted across 13 patients with a 68-year median age, all with chronic pain, and a 19-month median follow-up time. The procedures were performed on patients diagnosed with degenerative intervertebral disc disorders and underlying spinal stenosis. There were no complications, no revisions or any implant failures. In addition, post-op pain measured by the Numeric Pain Rating Scale showed statistically significant pain reduction for back and leg pain.
Dr. Pandey, President, and COO of Inspan, said, “Inspan has a long clinical track record since 2010, and we have continuously incorporated feedback from our doctors to improve the device, instruments, and technique. We will continue our commitment to innovation and listen to our doctors.”
Source: Inspan

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







