
 Copy to clipboard
Copy to clipboard 
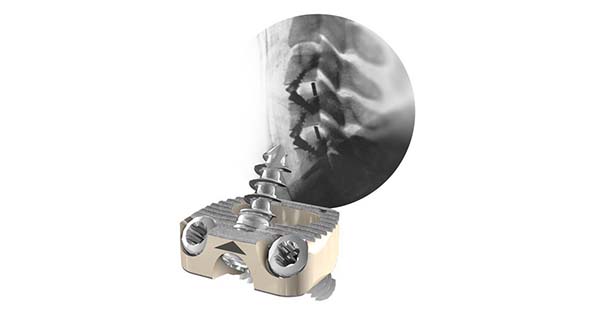
Centinel Spine announced the recent publication of a research study on its STALIF C-Ti® product in the Journal of Surgery & Anesthesia Research: “Preliminary 12-Month Safety and Efficacy Outcomes for the Treatment of Cervical Radiculopathy and Myelopathy with the Stalif-C Integrated Interbody Fusion Device.” The prospective multi-center study supports that patients receiving STALIF C-Ti implants demonstrated significant improvements in clinical outcome scores with minimal overall complication rate.
The clinical study utilizing the STALIF C-Ti integrated cage-screw implants was performed in 145 patients and 12-month outcome scores demonstrated significant improvement in all available patient reported outcome scores collected, including NDI, VAS neck, VAS left arm and VAS right arm.
The STALIF C-Ti implant technology has been engineered based on the STALIF design, which has a history of over 30 years of clinical usage and has helped thousands of patients regain their lives. STALIF implants provide compressive fixation at the fusion site, pulling the vertebral bodies onto the implant and graft material to enhance opportunities for fusion in line with Wolff’s Law of Bone Healing.
Centinel Spine’s CEO, Steve Murray, stated, “We are committed to providing clinical evidence to support our portfolio of devices and this study is one of the few multi-center prospective clinical evaluations of this type of technology. We are dedicated to advancing evidence that further supports the stand-alone fusion platform that was pioneered by Centinel Spine.”
Source: Centinel Spine, LLC
Centinel Spine announced the recent publication of a research study on its STALIF C-Ti® product in the Journal of Surgery & Anesthesia Research: "Preliminary 12-Month Safety and Efficacy Outcomes for the Treatment of Cervical Radiculopathy and Myelopathy with the Stalif-C Integrated Interbody Fusion Device." The prospective multi-center study...
Centinel Spine announced the recent publication of a research study on its STALIF C-Ti® product in the Journal of Surgery & Anesthesia Research: “Preliminary 12-Month Safety and Efficacy Outcomes for the Treatment of Cervical Radiculopathy and Myelopathy with the Stalif-C Integrated Interbody Fusion Device.” The prospective multi-center study supports that patients receiving STALIF C-Ti implants demonstrated significant improvements in clinical outcome scores with minimal overall complication rate.
The clinical study utilizing the STALIF C-Ti integrated cage-screw implants was performed in 145 patients and 12-month outcome scores demonstrated significant improvement in all available patient reported outcome scores collected, including NDI, VAS neck, VAS left arm and VAS right arm.
The STALIF C-Ti implant technology has been engineered based on the STALIF design, which has a history of over 30 years of clinical usage and has helped thousands of patients regain their lives. STALIF implants provide compressive fixation at the fusion site, pulling the vertebral bodies onto the implant and graft material to enhance opportunities for fusion in line with Wolff’s Law of Bone Healing.
Centinel Spine’s CEO, Steve Murray, stated, “We are committed to providing clinical evidence to support our portfolio of devices and this study is one of the few multi-center prospective clinical evaluations of this type of technology. We are dedicated to advancing evidence that further supports the stand-alone fusion platform that was pioneered by Centinel Spine.”
Source: Centinel Spine, LLC

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







