
 Copy to clipboard
Copy to clipboard 
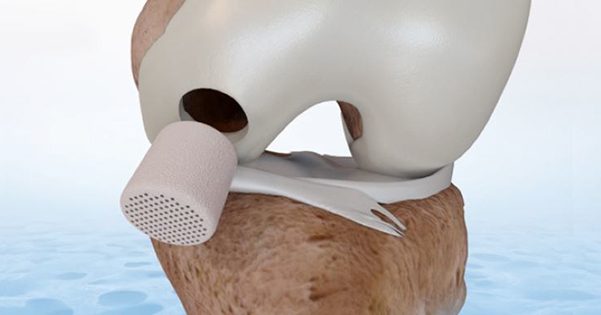
CartiHeal announced two-year results of a pivotal Investigational Device Exemption study (IDE). The study was conducted to test the potential superiority of the Agili-C™ implant vs. microfracture and debridement for the treatment of joint surface lesions, chondral and osteochondral defects, in the knee joint. The IDE study is a multicenter, 2:1 randomized, open-labeled and controlled trial. 251 Subjects, 167 in the Agili-C™ arm and 84 in the SSOC arm, were enrolled in 26 sites in the U.S. and outside the U.S.
The primary endpoint for this study was the change from baseline to 24 months in average of the 5 subscales of the Knee injury and Osteoarthritis Outcome Score (KOOS Overall): Pain, Other Symptoms, Quality of Life – QOL, Activities of Daily Living – ADL and Sports. The KOOS Overall Score ranges from 0 to 100, where higher values represent better outcomes.
The data generated from this trial demonstrated superiority of Agili-C to the current surgical standard of care (debridement or microfracture). The Bayesian posterior probability of superiority at Month 24 was determined to be 1.000, exceeding the prespecified threshold of 0.98 required to demonstrate superiority.
“Study results, which demonstrate the superiority of the Agili-C implant over the current surgical standard of care, offers an important potential benefit, as reflected in our Breakthrough Designation by the FDA, for patients who lack other sufficient treatment options,” said Nir Altschuler, CartiHeal’s founder and CEO. “We are looking forward to working closely with the FDA and plan to submit the PMA application later this year.”
Source: CartiHeal
CartiHeal announced two-year results of a pivotal Investigational Device Exemption study (IDE). The study was conducted to test the potential superiority of the Agili-C™ implant vs. microfracture and debridement for the treatment of joint surface lesions, chondral and osteochondral defects, in the knee joint. The IDE study is a multicenter, 2:1...
CartiHeal announced two-year results of a pivotal Investigational Device Exemption study (IDE). The study was conducted to test the potential superiority of the Agili-C™ implant vs. microfracture and debridement for the treatment of joint surface lesions, chondral and osteochondral defects, in the knee joint. The IDE study is a multicenter, 2:1 randomized, open-labeled and controlled trial. 251 Subjects, 167 in the Agili-C™ arm and 84 in the SSOC arm, were enrolled in 26 sites in the U.S. and outside the U.S.
The primary endpoint for this study was the change from baseline to 24 months in average of the 5 subscales of the Knee injury and Osteoarthritis Outcome Score (KOOS Overall): Pain, Other Symptoms, Quality of Life – QOL, Activities of Daily Living – ADL and Sports. The KOOS Overall Score ranges from 0 to 100, where higher values represent better outcomes.
The data generated from this trial demonstrated superiority of Agili-C to the current surgical standard of care (debridement or microfracture). The Bayesian posterior probability of superiority at Month 24 was determined to be 1.000, exceeding the prespecified threshold of 0.98 required to demonstrate superiority.
“Study results, which demonstrate the superiority of the Agili-C implant over the current surgical standard of care, offers an important potential benefit, as reflected in our Breakthrough Designation by the FDA, for patients who lack other sufficient treatment options,” said Nir Altschuler, CartiHeal’s founder and CEO. “We are looking forward to working closely with the FDA and plan to submit the PMA application later this year.”
Source: CartiHeal

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







