
 Copy to clipboard
Copy to clipboard 
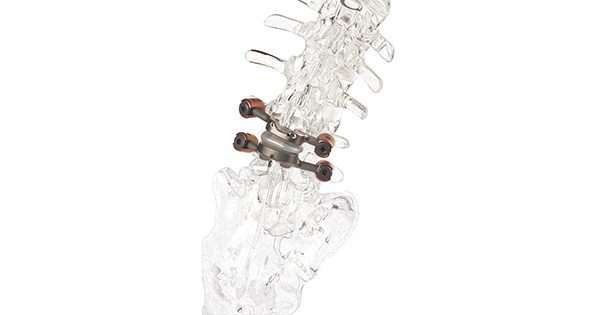
Premia Spine released results of the TOPS System pivotal study, Lumbar Facet Arthroplasty Versus Fusion for Grade-I Degenerative Spondylolisthesis with Stenosis: A Prospective Randomized Controlled Trial.
The study compares the effectiveness of decompression plus lumbar facet arthroplasty versus decompression plus lumbar spinal fusion in patients with lumbar spinal stenosis and Grade I degenerative spondylolisthesis. Conducted under an FDA Investigational Device Exemption, this trial represents a significant advancement in spinal treatment options.
Key findings include:
- Superior Clinical Success: The study demonstrated that 73.5% of patients in the arthroplasty group achieved composite clinical success at 24 months postoperatively compared to just 25.5% in the fusion group (p < 0.001). This reflects a substantial between-group difference of 47.9% (95% confidence interval, 33.0% to 62.8%).
- Patient-Reported Outcomes: The arthroplasty group outperformed the fusion group in most patient-reported outcome measures, including the Oswestry Disability Index, visual analog scale for back pain, and Zurich Claudication Questionnaire scores, all at 24 months postoperatively.
- Reduced Complications: The fusion group exhibited a higher rate of symptomatic adjacent segment degeneration and had a higher revision rate. Surgical variables and overall complications were similar between the groups.
This randomized controlled trial included 321 patients who were assigned in a 2:1 ratio to undergo either decompression plus lumbar facet arthroplasty or decompression plus lumbar fusion. The primary outcome was the overall rate of composite clinical success at 24 months postoperatively. Secondary outcomes included ODI scores, VAS for back and leg pain, ZCQ scores, Short Form (SF)-12 scores, radiographic parameters, surgical variables and complications.
Patients eligible for inclusion had LSS and DS, were between 35 and 80 years of age, and had undergone more than six months of unsuccessful nonsurgical therapy. The trial was conducted at 37 medical centers with institutional review board approval and patient consent.
Peter Wehrly, President of Premia Spine, added, “This study marks a significant milestone in spinal treatment. The TOPS System offers a viable alternative to traditional fusion, preserving natural spinal motion and reducing the risk of adjacent segment degeneration.”
Source: Premia Spine
Premia Spine released results of the TOPS System pivotal study, Lumbar Facet Arthroplasty Versus Fusion for Grade-I Degenerative Spondylolisthesis with Stenosis: A Prospective Randomized Controlled Trial.
The study compares the effectiveness of decompression plus lumbar facet arthroplasty versus decompression plus lumbar spinal fusion in...
Premia Spine released results of the TOPS System pivotal study, Lumbar Facet Arthroplasty Versus Fusion for Grade-I Degenerative Spondylolisthesis with Stenosis: A Prospective Randomized Controlled Trial.
The study compares the effectiveness of decompression plus lumbar facet arthroplasty versus decompression plus lumbar spinal fusion in patients with lumbar spinal stenosis and Grade I degenerative spondylolisthesis. Conducted under an FDA Investigational Device Exemption, this trial represents a significant advancement in spinal treatment options.
Key findings include:
- Superior Clinical Success: The study demonstrated that 73.5% of patients in the arthroplasty group achieved composite clinical success at 24 months postoperatively compared to just 25.5% in the fusion group (p < 0.001). This reflects a substantial between-group difference of 47.9% (95% confidence interval, 33.0% to 62.8%).
- Patient-Reported Outcomes: The arthroplasty group outperformed the fusion group in most patient-reported outcome measures, including the Oswestry Disability Index, visual analog scale for back pain, and Zurich Claudication Questionnaire scores, all at 24 months postoperatively.
- Reduced Complications: The fusion group exhibited a higher rate of symptomatic adjacent segment degeneration and had a higher revision rate. Surgical variables and overall complications were similar between the groups.
This randomized controlled trial included 321 patients who were assigned in a 2:1 ratio to undergo either decompression plus lumbar facet arthroplasty or decompression plus lumbar fusion. The primary outcome was the overall rate of composite clinical success at 24 months postoperatively. Secondary outcomes included ODI scores, VAS for back and leg pain, ZCQ scores, Short Form (SF)-12 scores, radiographic parameters, surgical variables and complications.
Patients eligible for inclusion had LSS and DS, were between 35 and 80 years of age, and had undergone more than six months of unsuccessful nonsurgical therapy. The trial was conducted at 37 medical centers with institutional review board approval and patient consent.
Peter Wehrly, President of Premia Spine, added, “This study marks a significant milestone in spinal treatment. The TOPS System offers a viable alternative to traditional fusion, preserving natural spinal motion and reducing the risk of adjacent segment degeneration.”
Source: Premia Spine

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







