
 Copy to clipboard
Copy to clipboard 
Providence Medical Technology announced publication of initial FUSE study results in the journal Spine. The journal presents peer-reviewed results of a pivotal, multicenter randomized controlled trial showing that tissue-sparing circumferential cervical fusion (CCF) significantly outperforms anterior cervical discectomy and fusion (ACDF) alone in treating three-level degenerative disc disease (DDD).
The article, “Three-level anterior cervical discectomy and fusion with or without an investigational posterior stabilization system assessed through 24 months: a multi-center randomized controlled trial,” represents the first peer-reviewed results from the FUSE IDE trial, which enrolled 227 patients across 18 U.S. sites and compared outcomes between standard three-level ACDF and ADCF with Providence’s CORUS Posterior Cervical Stabilization System (PCSS). Results demonstrated statistically significant improvements in the CCF arm in fusion success, safety, and the reduction of reoperation rates without adding procedural risk or recovery burden.
Key findings include:
- Superior Fusion Success at 12 Months: CCF achieved a 61.0% composite fusion success rate compared to just 17% in the ACDF group—a nearly fourfold improvement (p<0.001). Fusion was strictly defined by <2° motion at all levels on flexion-extension radiographs and contiguous bridging bone on thin-slice CT at each level, verified by independent imaging reviewers. At the final 24-month follow-up, 75% of CCF patients achieved fusion versus 33% of ACDF patients.
- Fewer Reoperations within 24 Months: The need for revision surgery was more than ten times lower in the CCF group (2% vs. 23%, p<0.001).
- Improved Composite Safety: CCF showed superior overall safety success at 2 years (50.8% vs. 22.8%, p<0.002), based on fusion success, absence of revision, neurological maintenance or improvement, and NDI score gains. Despite being a circumferential procedure, CCF demonstrated statistically fewer procedure-related complications than ACDF alone (p=0.005), with only 10cc of added blood loss and 98 minutes of additional operative time on average.
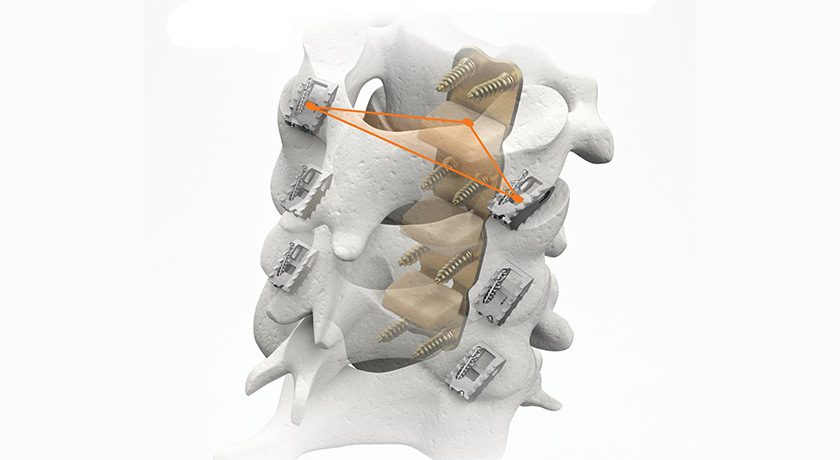
CORUS PCSS, now FDA-cleared for use in up to three cervical levels, is designed to achieve posterior fixation with minimal disruption to muscle and soft tissue. Its unique design enables stabilization across the facet joints through a posterior approach, avoiding the high morbidity associated with traditional open posterior fixation.
The FUSE study was conducted under FDA oversight as part of an Investigational Device Exemption and formed the clinical basis for FDA clearance of the CORUS PCSS system in July 2024.
“The publication of the FUSE study in Spine represents a major milestone for the field of cervical spine surgery,” said Jeff Smith, CEO of Providence Medical Technology. “The science is clear: tissue-sparing CCF with CORUS PCSS is safer, more effective, and more durable than ACDF alone in multilevel cervical fusion cases. As such, these results should prompt spine surgeons and payors alike to reconsider how we treat multilevel cervical DDD.”
Source: Providence Medical Technology
Providence Medical Technology announced publication of initial FUSE study results in the journal Spine. The journal presents peer-reviewed results of a pivotal, multicenter randomized controlled trial showing that tissue-sparing circumferential cervical fusion (CCF) significantly outperforms anterior cervical discectomy and fusion (ACDF) alone in...
Providence Medical Technology announced publication of initial FUSE study results in the journal Spine. The journal presents peer-reviewed results of a pivotal, multicenter randomized controlled trial showing that tissue-sparing circumferential cervical fusion (CCF) significantly outperforms anterior cervical discectomy and fusion (ACDF) alone in treating three-level degenerative disc disease (DDD).
The article, “Three-level anterior cervical discectomy and fusion with or without an investigational posterior stabilization system assessed through 24 months: a multi-center randomized controlled trial,” represents the first peer-reviewed results from the FUSE IDE trial, which enrolled 227 patients across 18 U.S. sites and compared outcomes between standard three-level ACDF and ADCF with Providence’s CORUS Posterior Cervical Stabilization System (PCSS). Results demonstrated statistically significant improvements in the CCF arm in fusion success, safety, and the reduction of reoperation rates without adding procedural risk or recovery burden.
Key findings include:
- Superior Fusion Success at 12 Months: CCF achieved a 61.0% composite fusion success rate compared to just 17% in the ACDF group—a nearly fourfold improvement (p<0.001). Fusion was strictly defined by <2° motion at all levels on flexion-extension radiographs and contiguous bridging bone on thin-slice CT at each level, verified by independent imaging reviewers. At the final 24-month follow-up, 75% of CCF patients achieved fusion versus 33% of ACDF patients.
- Fewer Reoperations within 24 Months: The need for revision surgery was more than ten times lower in the CCF group (2% vs. 23%, p<0.001).
- Improved Composite Safety: CCF showed superior overall safety success at 2 years (50.8% vs. 22.8%, p<0.002), based on fusion success, absence of revision, neurological maintenance or improvement, and NDI score gains. Despite being a circumferential procedure, CCF demonstrated statistically fewer procedure-related complications than ACDF alone (p=0.005), with only 10cc of added blood loss and 98 minutes of additional operative time on average.
CORUS PCSS, now FDA-cleared for use in up to three cervical levels, is designed to achieve posterior fixation with minimal disruption to muscle and soft tissue. Its unique design enables stabilization across the facet joints through a posterior approach, avoiding the high morbidity associated with traditional open posterior fixation.
The FUSE study was conducted under FDA oversight as part of an Investigational Device Exemption and formed the clinical basis for FDA clearance of the CORUS PCSS system in July 2024.
“The publication of the FUSE study in Spine represents a major milestone for the field of cervical spine surgery,” said Jeff Smith, CEO of Providence Medical Technology. “The science is clear: tissue-sparing CCF with CORUS PCSS is safer, more effective, and more durable than ACDF alone in multilevel cervical fusion cases. As such, these results should prompt spine surgeons and payors alike to reconsider how we treat multilevel cervical DDD.”
Source: Providence Medical Technology

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.