
 Copy to clipboard
Copy to clipboard 
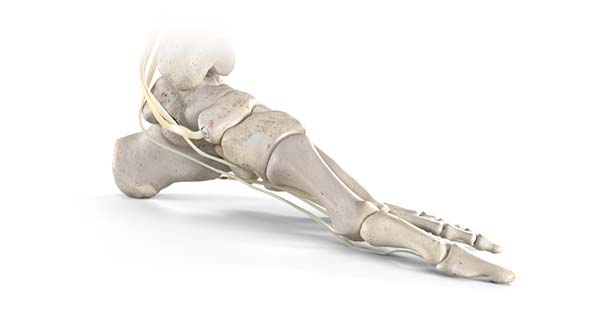
Stryker’s Trauma & Extremities division has launched its Citrelock™ Tendon Fixation Device System. Citrelock provides a differentiated design via a tendon thread featuring a resorbable technology, known as Citregen™, that has unique chemical and mechanical properties for orthopedic surgical applications.
Citrelock Tendon Fixation Device System offers:
- A controlled and homogeneous resorption process that prevents bulk degradation and chronic inflammation.
- Compressive strength that is comparable to cortical bone with a modulus comparable to cancellous bone.
- Citregen contains citrate, calcium and phosphate molecules that are inherent to the bone anatomy.
- Material polymer structure that mimics the extracellular matrix protein network.
Citregen maintains structural integrity during the healing phase, while the implant is replaced by host tissue over time.
Source: Stryker
Stryker’s Trauma & Extremities division has launched its Citrelock™ Tendon Fixation Device System. Citrelock provides a differentiated design via a tendon thread featuring a resorbable technology, known as Citregen™, that has unique chemical and mechanical properties for orthopedic surgical applications.
Citrelock Tendon Fixation Device...
Stryker’s Trauma & Extremities division has launched its Citrelock™ Tendon Fixation Device System. Citrelock provides a differentiated design via a tendon thread featuring a resorbable technology, known as Citregen™, that has unique chemical and mechanical properties for orthopedic surgical applications.
Citrelock Tendon Fixation Device System offers:
- A controlled and homogeneous resorption process that prevents bulk degradation and chronic inflammation.
- Compressive strength that is comparable to cortical bone with a modulus comparable to cancellous bone.
- Citregen contains citrate, calcium and phosphate molecules that are inherent to the bone anatomy.
- Material polymer structure that mimics the extracellular matrix protein network.
Citregen maintains structural integrity during the healing phase, while the implant is replaced by host tissue over time.
Source: Stryker

You’ve reached your limit.
We’re glad you’re finding value in our content — and we’d love for you to keep going.
Subscribe now for unlimited access to orthopedic business intelligence.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







