
 Copy to clipboard
Copy to clipboard 
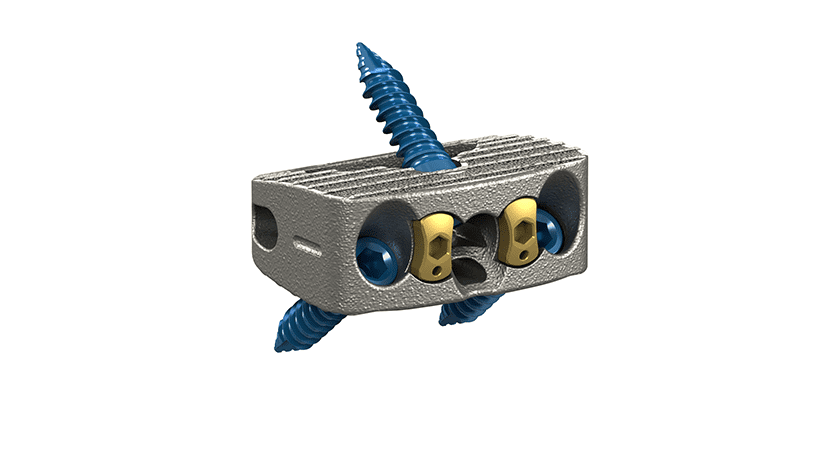
Stryker released the Monterey AL Interbody, a stand-alone interbody fusion device designed for anterior lumbar interbody fusion. Monterey AL comprises both solid and porous structures within a single implant, leveraging Stryker’s Tritanium In-Growth Technology, a material designed to mimic cancellous bone and provide an environment favorable to bone regeneration and fusion. New data demonstrates that undifferentiated stem cells grown on Tritanium exhibited osteogenic Alkaline Phosphatase without requiring growth factor supplements.
Monterey AL Interbody System highlights:
- Tritanium In-Growth Technology: Tritanium is a 3D-printed, novel, highly porous titanium material designed for bone in-growth and biological fixation, built using AMagine, Stryker’s proprietary approach to implant creation using additive manufacturing.
- Functionally optimized footprint options: Strategically deeper and narrower cage footprints allow surgeons to create indirect decompression by distracting the disc space posteriorly. These geometries are designed to both help prevent the cage from impinging posteriorly into the neural foramen and lessen the need to countersink the cage, thereby allowing for easy access to the anterior screw holes.
- Straightforward instruments: The robust medial attachment, multiple technique possibilities, and a wide variety of screwdriver options are designed to facilitate clear visualization of and easy access to the surgical site once the approach is complete and a retractor is in place.
Robbie Robinson, President of the Stryker Spine division, said, “Monterey AL combines more than 20 years of expertise in the creation of porous materials using additive manufacturing with innovative implants and instruments that are designed to give surgeons the flexibility to use our system without having to alter their preferred technique.”
Source: Stryker
Stryker released the Monterey AL Interbody, a stand-alone interbody fusion device designed for anterior lumbar interbody fusion. Monterey AL comprises both solid and porous structures within a single implant, leveraging Stryker’s Tritanium In-Growth Technology, a material designed to mimic cancellous bone and provide an environment favorable to...
Stryker released the Monterey AL Interbody, a stand-alone interbody fusion device designed for anterior lumbar interbody fusion. Monterey AL comprises both solid and porous structures within a single implant, leveraging Stryker’s Tritanium In-Growth Technology, a material designed to mimic cancellous bone and provide an environment favorable to bone regeneration and fusion. New data demonstrates that undifferentiated stem cells grown on Tritanium exhibited osteogenic Alkaline Phosphatase without requiring growth factor supplements.
Monterey AL Interbody System highlights:
- Tritanium In-Growth Technology: Tritanium is a 3D-printed, novel, highly porous titanium material designed for bone in-growth and biological fixation, built using AMagine, Stryker’s proprietary approach to implant creation using additive manufacturing.
- Functionally optimized footprint options: Strategically deeper and narrower cage footprints allow surgeons to create indirect decompression by distracting the disc space posteriorly. These geometries are designed to both help prevent the cage from impinging posteriorly into the neural foramen and lessen the need to countersink the cage, thereby allowing for easy access to the anterior screw holes.
- Straightforward instruments: The robust medial attachment, multiple technique possibilities, and a wide variety of screwdriver options are designed to facilitate clear visualization of and easy access to the surgical site once the approach is complete and a retractor is in place.
Robbie Robinson, President of the Stryker Spine division, said, “Monterey AL combines more than 20 years of expertise in the creation of porous materials using additive manufacturing with innovative implants and instruments that are designed to give surgeons the flexibility to use our system without having to alter their preferred technique.”
Source: Stryker

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







