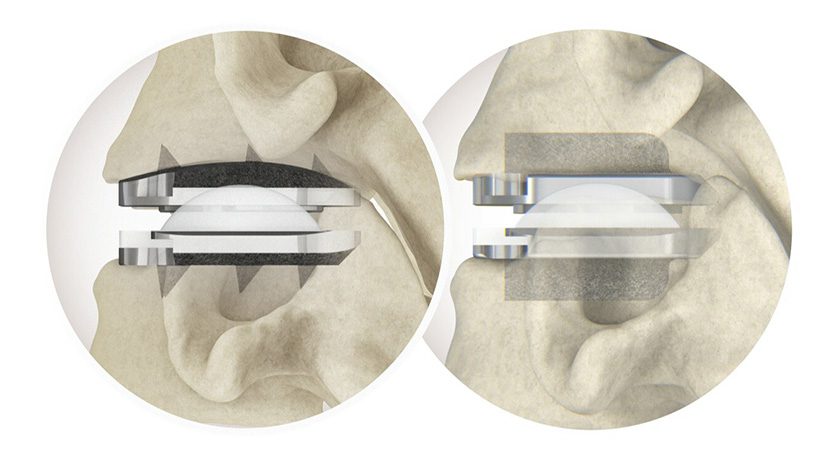
Stryker received FDA 510(k) clearance to market the SpineJack® implantable reduction system to treat osteoporotic vertebral compression fractures. Limited launch will occur throughout the end of 2018.
SpineJack comprises three titanium implants of differing diameters that address 95% of VCFs and all patient morphologies. The 30-minute, minimally invasive x-ray guided procedure is intended to support patient discharge shortly after surgery.
Stryker acquired the technology with its purchase of Vexim in 2017. SpineJack has been commercially available in Europe since 2008, with >70,000 units implanted worldwide.
Sources: Stryker; ORTHOWORLD Inc.
Stryker received FDA 510(k) clearance to market the SpineJack® implantable reduction system to treat osteoporotic vertebral compression fractures. Limited launch will occur throughout the end of 2018.
SpineJack comprises three titanium implants of differing diameters that address 95% of VCFs and all patient morphologies. The 30-minute,...
Stryker received FDA 510(k) clearance to market the SpineJack® implantable reduction system to treat osteoporotic vertebral compression fractures. Limited launch will occur throughout the end of 2018.
SpineJack comprises three titanium implants of differing diameters that address 95% of VCFs and all patient morphologies. The 30-minute, minimally invasive x-ray guided procedure is intended to support patient discharge shortly after surgery.
Stryker acquired the technology with its purchase of Vexim in 2017. SpineJack has been commercially available in Europe since 2008, with >70,000 units implanted worldwide.
Sources: Stryker; ORTHOWORLD Inc.


You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.