Copy to clipboard
Copy to clipboard 
Each month, ORTHOWORLD examines updates to FDA’s orthopaedic-related 510(k) database and highlights those products deemed strategic, defined as a company’s first 510(k) clearance, first clearance in a segment that is new to the company, clearance for a type of device not formerly marketed by a company, etc.
In the following list, companies receiving a first orthopaedic-related 510(k) are denoted with an asterisk.
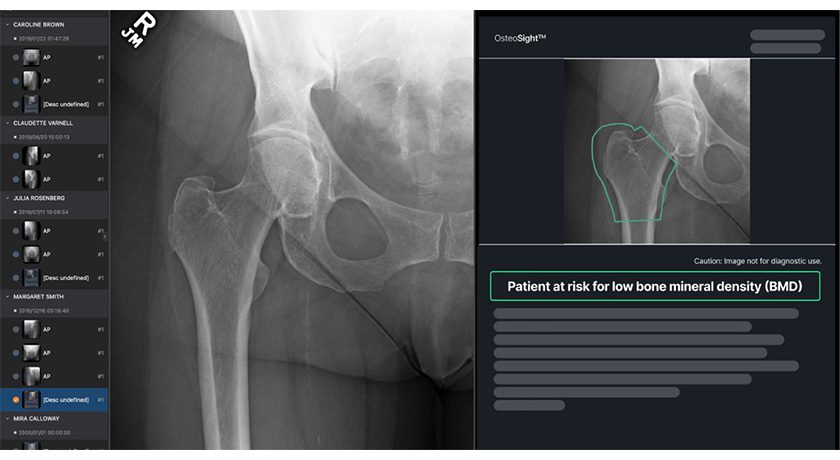
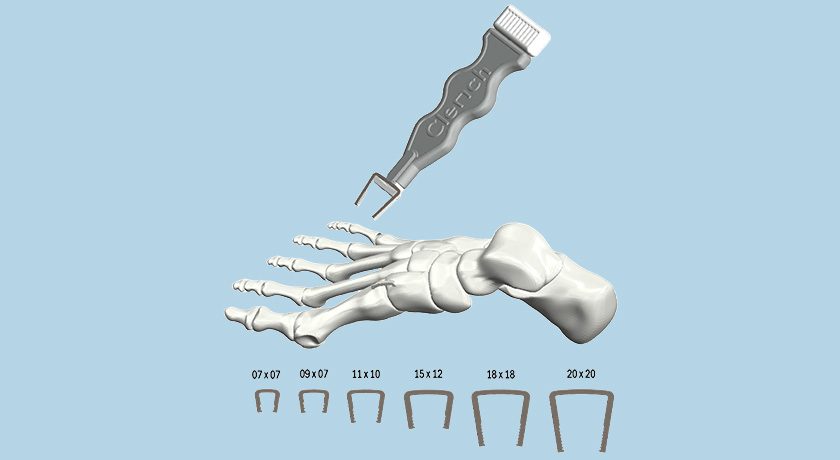
Envision 3D: Image Guidance System (7D Surgical*); Sphynx Anterolateral Plate (Eden Spine); Destiknee Total Knee (Meril Healthcare*); Hubble II Lumbar Interbody Fusion System (Orbbo Surgical*)
Each month, ORTHOWORLD examines updates to FDA's orthopaedic-related 510(k) database and highlights those products deemed strategic, defined as a company's first 510(k) clearance, first clearance in a segment that is new to the company, clearance for a type of device not formerly marketed by a company, etc.
In the following list, companies...
Each month, ORTHOWORLD examines updates to FDA’s orthopaedic-related 510(k) database and highlights those products deemed strategic, defined as a company’s first 510(k) clearance, first clearance in a segment that is new to the company, clearance for a type of device not formerly marketed by a company, etc.
In the following list, companies receiving a first orthopaedic-related 510(k) are denoted with an asterisk.
Envision 3D: Image Guidance System (7D Surgical*); Sphynx Anterolateral Plate (Eden Spine); Destiknee Total Knee (Meril Healthcare*); Hubble II Lumbar Interbody Fusion System (Orbbo Surgical*)

You’ve reached your limit.
We’re glad you’re finding value in our content — and we’d love for you to keep going.
Subscribe now for unlimited access to orthopedic business intelligence.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.