
 Copy to clipboard
Copy to clipboard 
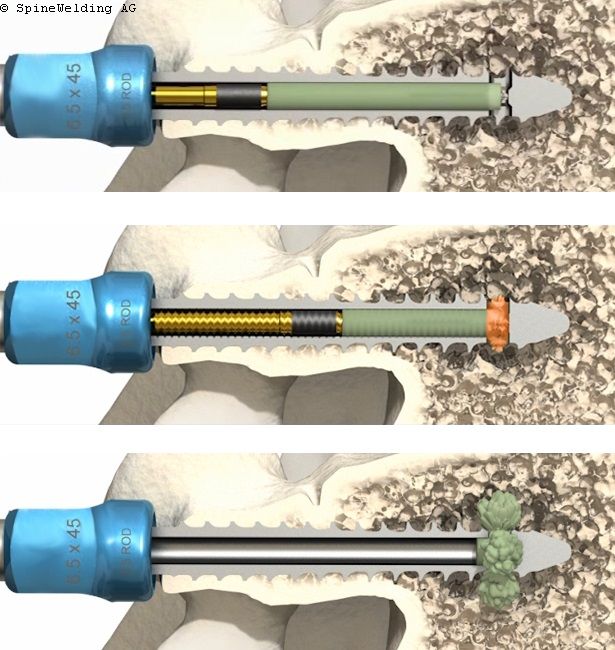
SpineWelding received FDA 510(k) clearance to market the Elaris Pedicle Screw, the company’s first 510(k) clearance. Elaris is indicated for the treatment of degenerative disc disease, spondylolisthesis, trauma, spinal stenosis, curvatures, tumor, pseudarthrosis and failed previous fusion. The cannulated pedicle screws are available in two diameters and four lengths.
Elaris is enabled by proprietary BoneWelding technology that is designed to support load-bearing implants. The process uses ultrasonic energy to liquefy pre-defined polymeric components of the Elaris Pin, extruding them through the screw’s fenestrations and forming a bond between implant and bone. The liquid polymer solidifies upon contact, forming a confined, solid ring around the screw tip. The bioresorbable polymer is gradually metabolized into H2O and carbon dioxide. This adjunctive fixation to individual screws does not disrupt case flow.
As opposed to screws without additional fixation, bench and animal testing have demonstrated that the polymer enhancement in Elaris provides appropriate dynamic pullout strength for up to 52 weeks as well as improved resistance to toggle loosening. The pedicle screws can be removed as needed after polymer enhancement.
Source: SpineWelding AG
SpineWelding received FDA 510(k) clearance to market the Elaris Pedicle Screw, the company’s first 510(k) clearance. Elaris is indicated for the treatment of degenerative disc disease, spondylolisthesis, trauma, spinal stenosis, curvatures, tumor, pseudarthrosis and failed previous fusion. The cannulated pedicle screws are available in two...
SpineWelding received FDA 510(k) clearance to market the Elaris Pedicle Screw, the company’s first 510(k) clearance. Elaris is indicated for the treatment of degenerative disc disease, spondylolisthesis, trauma, spinal stenosis, curvatures, tumor, pseudarthrosis and failed previous fusion. The cannulated pedicle screws are available in two diameters and four lengths.
Elaris is enabled by proprietary BoneWelding technology that is designed to support load-bearing implants. The process uses ultrasonic energy to liquefy pre-defined polymeric components of the Elaris Pin, extruding them through the screw’s fenestrations and forming a bond between implant and bone. The liquid polymer solidifies upon contact, forming a confined, solid ring around the screw tip. The bioresorbable polymer is gradually metabolized into H2O and carbon dioxide. This adjunctive fixation to individual screws does not disrupt case flow.
As opposed to screws without additional fixation, bench and animal testing have demonstrated that the polymer enhancement in Elaris provides appropriate dynamic pullout strength for up to 52 weeks as well as improved resistance to toggle loosening. The pedicle screws can be removed as needed after polymer enhancement.

Source: SpineWelding AG

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







