
 Copy to clipboard
Copy to clipboard 
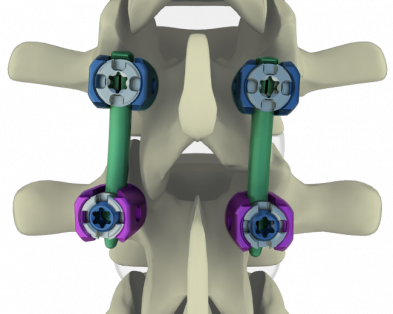 Spineway entered the Japan spine market with regulatory approval for Mont Blanc minimally invasive surgical devices to treat degenerative, deformative and traumatological diagnoses. Discussions with potential Japanese business partners are underway. Mont Blanc MIS line is currently distributed in 15 countries.
Spineway entered the Japan spine market with regulatory approval for Mont Blanc minimally invasive surgical devices to treat degenerative, deformative and traumatological diagnoses. Discussions with potential Japanese business partners are underway. Mont Blanc MIS line is currently distributed in 15 countries.
Earlier this year, the company announced its NEWWAY 2021 initiative to position the company as a key international player in minimally invasive surgery. Spineway is overhauling its marketing policy to address mature markets such as the U.S., Japan and Europe, strengthening its presence in these markets by partnering with Tier 1 distributors that will provide wider coverage in each territory.
Along with the reinforcement of its international presence, Spineway seeks to reposition its products on a more profitable market, like its Mont Blanc MIS line. The group will be alert to targeted partnership opportunities to accelerate expansion into certain countries or strengthen its position in areas where it is already present, like its partnership in China with Tinavi Medical for the development of implants and instruments adapted to the Tinavi robot.
Sources: Spineway
Spineway entered the Japan spine market with regulatory approval for Mont Blanc minimally invasive surgical devices to treat degenerative, deformative and traumatological diagnoses. Discussions with potential Japanese business partners are underway. Mont Blanc MIS line is currently distributed in 15 countries.
Earlier this year, the...
 Spineway entered the Japan spine market with regulatory approval for Mont Blanc minimally invasive surgical devices to treat degenerative, deformative and traumatological diagnoses. Discussions with potential Japanese business partners are underway. Mont Blanc MIS line is currently distributed in 15 countries.
Spineway entered the Japan spine market with regulatory approval for Mont Blanc minimally invasive surgical devices to treat degenerative, deformative and traumatological diagnoses. Discussions with potential Japanese business partners are underway. Mont Blanc MIS line is currently distributed in 15 countries.
Earlier this year, the company announced its NEWWAY 2021 initiative to position the company as a key international player in minimally invasive surgery. Spineway is overhauling its marketing policy to address mature markets such as the U.S., Japan and Europe, strengthening its presence in these markets by partnering with Tier 1 distributors that will provide wider coverage in each territory.
Along with the reinforcement of its international presence, Spineway seeks to reposition its products on a more profitable market, like its Mont Blanc MIS line. The group will be alert to targeted partnership opportunities to accelerate expansion into certain countries or strengthen its position in areas where it is already present, like its partnership in China with Tinavi Medical for the development of implants and instruments adapted to the Tinavi robot.
Sources: Spineway

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







