
 Copy to clipboard
Copy to clipboard 
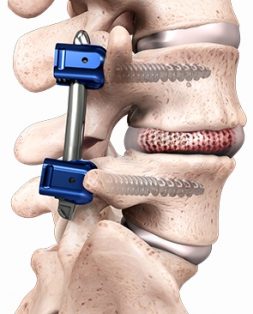
Two-year outcomes from Spineology’s SCOUT trial of the OptiMesh® device revealed substantial improvements in low back pain and function scores at 6, 12 and 24 months.
SCOUT (Spineology Clinical Outcomes Trial) is a 102-subject prospective, multi-center, non-randomized performance goal investigation designed to evaluating safety and effectiveness outcomes in instrumented lumbar interbody fusion procedures to treat degenerative disc disease. Eighty-two patients have completed 24-month follow-up, to date, showing a 98% fusion rate and 90% of subjects reporting “Excellent” or “Good” satisfaction.
The original 2003 FDA 510(k) clearance for OptiMesh supported graft containment inside the vertebral body. Data from SCOUT will support the submission of a De Novo application to FDA for expanded indications, allowing use with bone graft and supplemental posterior fixation in lumbar interbody fusion.
Two-year outcomes from Spineology's SCOUT trial of the OptiMesh® device revealed substantial improvements in low back pain and function scores at 6, 12 and 24 months.
SCOUT (Spineology Clinical Outcomes Trial) is a 102-subject prospective, multi-center, non-randomized performance goal investigation designed to evaluating safety and...
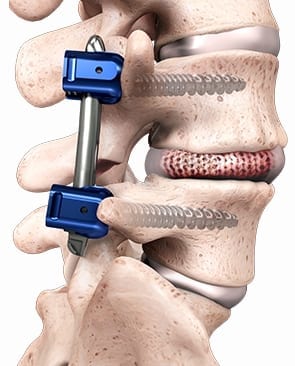
Two-year outcomes from Spineology’s SCOUT trial of the OptiMesh® device revealed substantial improvements in low back pain and function scores at 6, 12 and 24 months.
SCOUT (Spineology Clinical Outcomes Trial) is a 102-subject prospective, multi-center, non-randomized performance goal investigation designed to evaluating safety and effectiveness outcomes in instrumented lumbar interbody fusion procedures to treat degenerative disc disease. Eighty-two patients have completed 24-month follow-up, to date, showing a 98% fusion rate and 90% of subjects reporting “Excellent” or “Good” satisfaction.
The original 2003 FDA 510(k) clearance for OptiMesh supported graft containment inside the vertebral body. Data from SCOUT will support the submission of a De Novo application to FDA for expanded indications, allowing use with bone graft and supplemental posterior fixation in lumbar interbody fusion.

You’ve reached your limit.
We’re glad you’re finding value in our content — and we’d love for you to keep going.
Subscribe now for unlimited access to orthopedic business intelligence.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.