
 Copy to clipboard
Copy to clipboard 
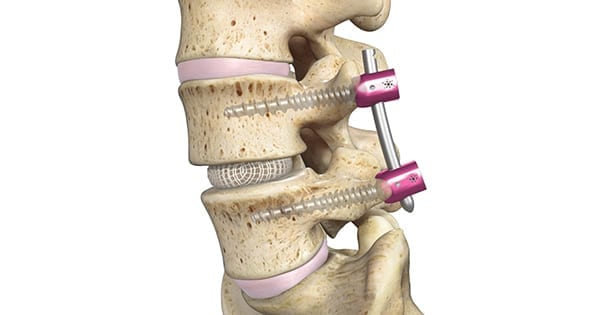
Spineology was granted de novo classification from FDA for the OptiMesh® expandable spinal interbody fusion system. The grant follows successful completion of the SCOUT (Spineology Clinical Outcomes Trial) Investigational Device Exemption trial, which revealed substantial improvements in low back pain and function scores at 6, 12 and 24 months.
OptiMesh expands in three dimensions, allowing surgeons to perform interbody fusion procedures through a 1 cm incision, reported to be the smallest access in the spine industry.
Product launch is slated for 1Q21 in lumbar fusion procedures.
“I am pleased to announce the De Novo grant of our OptiMesh implants and instrumentation to support the OptiLIF procedure…I anticipate OptiLIF will help take surgery for low back and leg pain to the next level through its ability to provide excellent patient outcomes, enhanced recovery and exceptional efficiency,” said John Booth, Spineology’s CEO.
Spineology was granted de novo classification from FDA for the OptiMesh® expandable spinal interbody fusion system. The grant follows successful completion of the SCOUT (Spineology Clinical Outcomes Trial) Investigational Device Exemption trial, which revealed substantial improvements in low back pain and function scores at 6, 12 and 24...
Spineology was granted de novo classification from FDA for the OptiMesh® expandable spinal interbody fusion system. The grant follows successful completion of the SCOUT (Spineology Clinical Outcomes Trial) Investigational Device Exemption trial, which revealed substantial improvements in low back pain and function scores at 6, 12 and 24 months.
OptiMesh expands in three dimensions, allowing surgeons to perform interbody fusion procedures through a 1 cm incision, reported to be the smallest access in the spine industry.
Product launch is slated for 1Q21 in lumbar fusion procedures.
“I am pleased to announce the De Novo grant of our OptiMesh implants and instrumentation to support the OptiLIF procedure…I anticipate OptiLIF will help take surgery for low back and leg pain to the next level through its ability to provide excellent patient outcomes, enhanced recovery and exceptional efficiency,” said John Booth, Spineology’s CEO.

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







