
 Copy to clipboard
Copy to clipboard 
Spineology completed enrollment of RaDical, its 2-year, 200-patient postmarket study of the Duo™ Expandable Interbody Fusion (IBF) System. RaDical is a prospective, Institutional Review Board-approved, multi-center study of lateral interbody fusion.
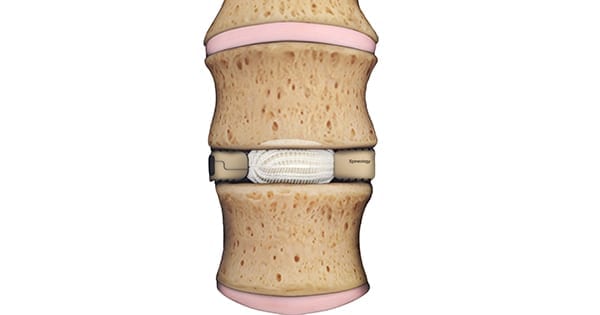
Duo Expandable is placed through an 18 mm portal tube, minimizing retraction of the neural structures and psoas muscle. Duo is filled with bone graft in situ to expand the device in all directions.
Data from the first 115 patients enrolled in RaDical demonstrated benefits to the patient, surgeon and hospital vs. traditional lateral interbody fusion systems, including:
- Reduced neurological deficits following surgery
- 33% decrease in operative time
- 37% shorter hospital stay
- Lower complication rate
John Booth, CEO, said, “Based on the data compiled to date, patients are experiencing long-term, clinically meaningful improvements in pain and function; high fusion rates; and fewer neurological deficits and complications compared to traditional lateral interbody fusion surgery. The data also shows a very positive economic impact to hospitals with shorter operative times and hospital stays compared to traditional lateral interbody fusion surgery. We look forward to collecting complete data on all 200 patients enrolled in the study and expect to see continued favorable outcomes, which will provide further evidence of the system’s benefits to patients, surgeons and hospitals.”
Spineology completed enrollment of RaDical, its 2-year, 200-patient postmarket study of the Duo™ Expandable Interbody Fusion (IBF) System. RaDical is a prospective, Institutional Review Board-approved, multi-center study of lateral interbody fusion.
Duo Expandable is placed through an 18 mm portal tube, minimizing retraction of the neural...
Spineology completed enrollment of RaDical, its 2-year, 200-patient postmarket study of the Duo™ Expandable Interbody Fusion (IBF) System. RaDical is a prospective, Institutional Review Board-approved, multi-center study of lateral interbody fusion.
Duo Expandable is placed through an 18 mm portal tube, minimizing retraction of the neural structures and psoas muscle. Duo is filled with bone graft in situ to expand the device in all directions.
Data from the first 115 patients enrolled in RaDical demonstrated benefits to the patient, surgeon and hospital vs. traditional lateral interbody fusion systems, including:
- Reduced neurological deficits following surgery
- 33% decrease in operative time
- 37% shorter hospital stay
- Lower complication rate
John Booth, CEO, said, “Based on the data compiled to date, patients are experiencing long-term, clinically meaningful improvements in pain and function; high fusion rates; and fewer neurological deficits and complications compared to traditional lateral interbody fusion surgery. The data also shows a very positive economic impact to hospitals with shorter operative times and hospital stays compared to traditional lateral interbody fusion surgery. We look forward to collecting complete data on all 200 patients enrolled in the study and expect to see continued favorable outcomes, which will provide further evidence of the system’s benefits to patients, surgeons and hospitals.”

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







