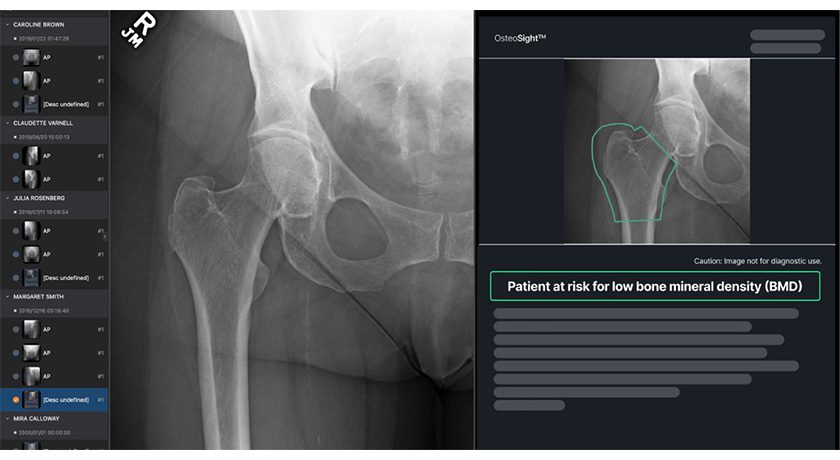

 Copy to clipboard
Copy to clipboard 
SpineGuard and XinRong Medical, expanded their collaboration with three concomitant agreements:
- China-exclusive distribution extension to the whole SpineGuard product portfolio
- Funding of the NMPA (National Medical Product Administration) registration process for SpineGuard’s new products via a cash injection by XinRong Medical in SpineGuard capital
- Potential co-development of a Smart Screw system for the Chinese spinal surgery market
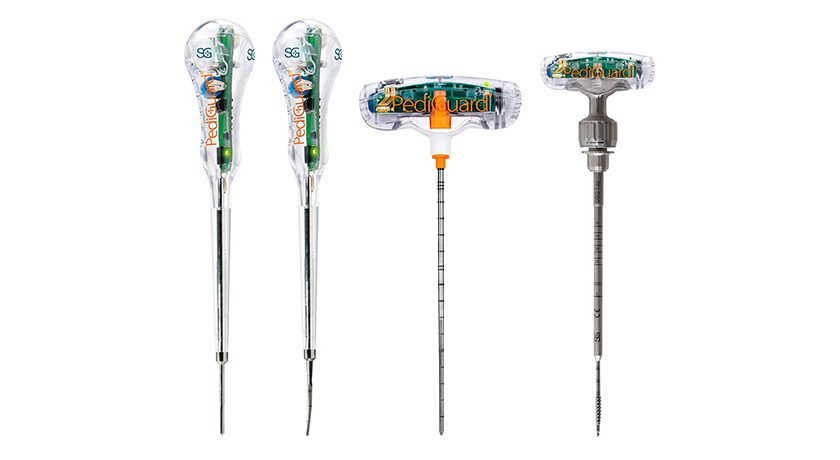
Until today, XinRong Medical was only selling SpineGuard’s Classic PediGuard products in China. This new distribution agreement will enable them to commercialize Curved, XS, Cannulated and Threaded versions of SpineGuard’s DSG (Dynamic Surgical Guidance) spinal drilling probes.
The NMPA registration of these new devices for the Chinese market will be funded via a €500K equity injection by XinRong Medical. SpineGuard will own all product registrations that will be executed through a third-party regulatory agent jointly appointed by both companies.
XinRong Medical and SpineGuard also decided to envision the co-development of a DSG enabled single step pedicle screw system with three key milestones triggering payments:
1) evaluation of the registration process and market access path
2) product design and submission of the registration dossier
3) product clearance
Source: SpineGuard
SpineGuard and XinRong Medical, expanded their collaboration with three concomitant agreements:
China-exclusive distribution extension to the whole SpineGuard product portfolio
Funding of the NMPA (National Medical Product Administration) registration process for SpineGuard's new products via a cash injection by XinRong Medical in...
SpineGuard and XinRong Medical, expanded their collaboration with three concomitant agreements:
- China-exclusive distribution extension to the whole SpineGuard product portfolio
- Funding of the NMPA (National Medical Product Administration) registration process for SpineGuard’s new products via a cash injection by XinRong Medical in SpineGuard capital
- Potential co-development of a Smart Screw system for the Chinese spinal surgery market
Until today, XinRong Medical was only selling SpineGuard’s Classic PediGuard products in China. This new distribution agreement will enable them to commercialize Curved, XS, Cannulated and Threaded versions of SpineGuard’s DSG (Dynamic Surgical Guidance) spinal drilling probes.
The NMPA registration of these new devices for the Chinese market will be funded via a €500K equity injection by XinRong Medical. SpineGuard will own all product registrations that will be executed through a third-party regulatory agent jointly appointed by both companies.
XinRong Medical and SpineGuard also decided to envision the co-development of a DSG enabled single step pedicle screw system with three key milestones triggering payments:
1) evaluation of the registration process and market access path
2) product design and submission of the registration dossier
3) product clearance
Source: SpineGuard

You’ve reached your limit.
We’re glad you’re finding value in our content — and we’d love for you to keep going.
Subscribe now for unlimited access to orthopedic business intelligence.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.