Copy to clipboard
Copy to clipboard 
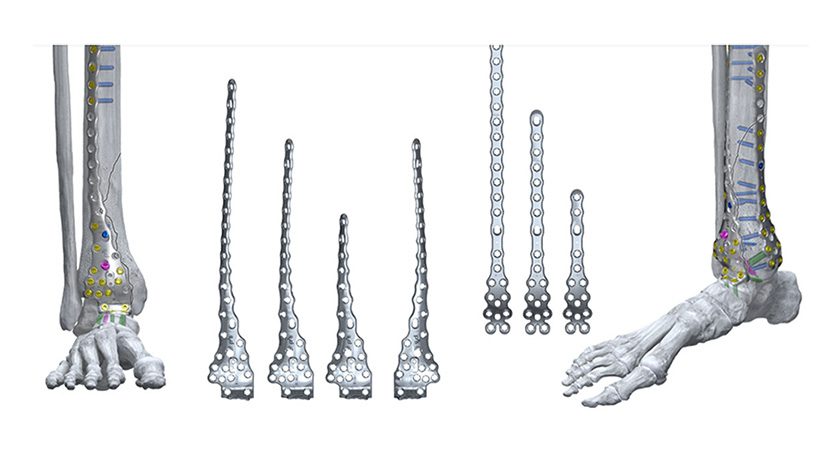
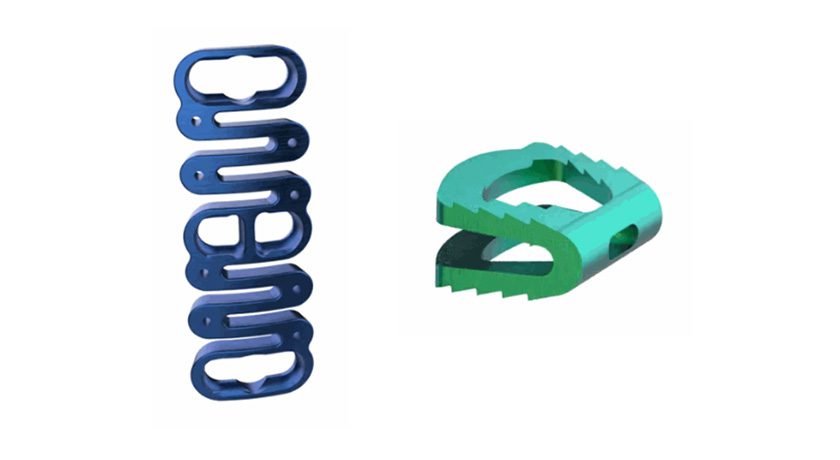
Spineart announces the full-market launch of its SCARLET AC-Ti secured anterior cervical cage in the United States following FDA 510(k) clearance in 2024.
SCARLET AC-Ti builds on a decade of experience with the SCARLET system and utilizes Ti-LIFE technology, a proprietary additive manufacturing process, providing a porous structure that closely mimics the trabecular bone structure. The secured anterior cervical cage is known for its redefined anatomical shape, integrated screw guides and anti-backout mechanism.
Source: Spineart
Spineart announces the full-market launch of its SCARLET AC-Ti secured anterior cervical cage in the United States following FDA 510(k) clearance in 2024.
SCARLET AC-Ti builds on a decade of experience with the SCARLET system and utilizes Ti-LIFE technology, a proprietary additive manufacturing process, providing a porous structure that...
Spineart announces the full-market launch of its SCARLET AC-Ti secured anterior cervical cage in the United States following FDA 510(k) clearance in 2024.
SCARLET AC-Ti builds on a decade of experience with the SCARLET system and utilizes Ti-LIFE technology, a proprietary additive manufacturing process, providing a porous structure that closely mimics the trabecular bone structure. The secured anterior cervical cage is known for its redefined anatomical shape, integrated screw guides and anti-backout mechanism.
Source: Spineart

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.