
 Copy to clipboard
Copy to clipboard 
Spinal Stabilization Technologies (SST) and BlueRiver Acquisition Corp. entered into a definitive business combination agreement. Upon the closing of the proposed transaction, the combined company will operate as Spinal Stabilization Technologies and be listed on an approved stock exchange.
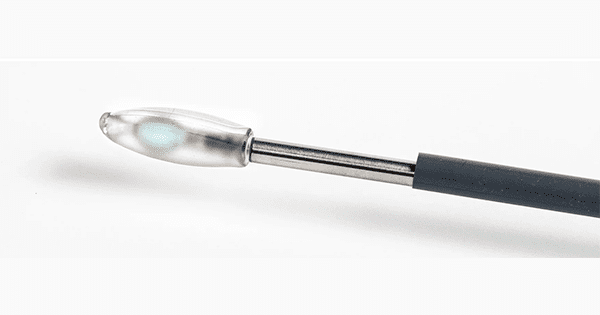
Spinal Stabilization Technologies is focused on development and commercialization of a proprietary lumbar implant for nucleus pulposus replacement to alleviate certain types of lower back pain. SST’s flagship product, PerQdisc, is a lumbar intervertebral disc nucleus replacement.
PerQdisc, a silicone-based prosthesis formed in situ, is designed to emulate the natural function of the native nucleus pulposus, providing a motion-preserving surgical solution.
In 2021, FDA designated the PerQdisc Nucleus Replacement System as a Breakthrough Device.
Data from the clinical trial suggests that PerQdisc may be a preferred surgical option for patients with mild to moderate disc degeneration and with severe back pain who have failed conservative therapy and for patients with a disc herniation requiring a discectomy procedure. These patients are usually not candidates for spinal fusion. As such, PerQdisc is designed to offer a less invasive treatment option compared to other surgical treatments.
A U.S. pivotal trial pursuant to an Investigational Device Exemption is expected to begin in 2Q24.
The combined company would have an estimated post-transaction enterprise value of approximately $302 million, assuming a proposed future $40.0 million equity raise and assuming 100% redemptions by BlueRiver public shareholders. Proceeds from the transaction, if any, will be used to execute on the company’s business plan, including funding the FDA pivotal trial and commercial expansion.
Spinal Stabilization Technologies (SST) and BlueRiver Acquisition Corp. entered into a definitive business combination agreement. Upon the closing of the proposed transaction, the combined company will operate as Spinal Stabilization Technologies and be listed on an approved stock exchange.
Spinal Stabilization Technologies is focused on...
Spinal Stabilization Technologies (SST) and BlueRiver Acquisition Corp. entered into a definitive business combination agreement. Upon the closing of the proposed transaction, the combined company will operate as Spinal Stabilization Technologies and be listed on an approved stock exchange.
Spinal Stabilization Technologies is focused on development and commercialization of a proprietary lumbar implant for nucleus pulposus replacement to alleviate certain types of lower back pain. SST’s flagship product, PerQdisc, is a lumbar intervertebral disc nucleus replacement.
PerQdisc, a silicone-based prosthesis formed in situ, is designed to emulate the natural function of the native nucleus pulposus, providing a motion-preserving surgical solution.
In 2021, FDA designated the PerQdisc Nucleus Replacement System as a Breakthrough Device.
Data from the clinical trial suggests that PerQdisc may be a preferred surgical option for patients with mild to moderate disc degeneration and with severe back pain who have failed conservative therapy and for patients with a disc herniation requiring a discectomy procedure. These patients are usually not candidates for spinal fusion. As such, PerQdisc is designed to offer a less invasive treatment option compared to other surgical treatments.
A U.S. pivotal trial pursuant to an Investigational Device Exemption is expected to begin in 2Q24.
The combined company would have an estimated post-transaction enterprise value of approximately $302 million, assuming a proposed future $40.0 million equity raise and assuming 100% redemptions by BlueRiver public shareholders. Proceeds from the transaction, if any, will be used to execute on the company’s business plan, including funding the FDA pivotal trial and commercial expansion.
Source: Spinal Stabilization Technologies

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







