
 Copy to clipboard
Copy to clipboard 
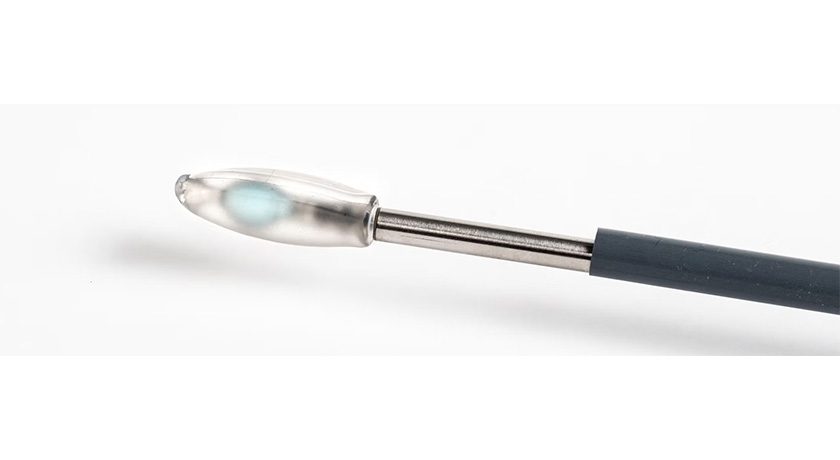
Spinal Stabilization Technologies enrolled the first U.S. patient in its Investigational Device Exemption (IDE) clinical trial for the PerQdisc Nucleus Replacement Device.
The IDE trial will assess the PerQdisc’s ability to replace the nucleus pulposus of a single lumbar disc (L1-L5) with the intent to preserve motion and alleviate pain. Currently, patients with painful lumbar DDD face limited surgical options, with many resorting to spinal fusion, which restricts motion and places increased stresses on adjacent spinal levels. SST’s PerQdisc is a breakthrough alternative that avoids fusion and leaves the surrounding anatomy intact.
To support the trial and accelerate its path toward regulatory approval, SST recently raised $17 million in funding from existing and new investors. This investment will advance the IDE trial and further clinical development initiatives.
“The reduction in pain and overall clinical outcomes observed with PerQdisc during the feasibility trials (over 40 patients treated) have been exceptional,” stated Dr. Pierce Nunley, Principal Investigator and Medical Director of the Spine Institute of Louisiana. “The procedure was notably straightforward, and the recovery period is anticipated to be brief. This technology holds the potential to revolutionize the treatment of patients suffering from discogenic low back pain, providing a straightforward, safe, and minimally invasive option that reduces pain and maintains mobility while restoring functionality.”
Spinal Stabilization Technologies enrolled the first U.S. patient in its Investigational Device Exemption (IDE) clinical trial for the PerQdisc Nucleus Replacement Device.
The IDE trial will assess the PerQdisc's ability to replace the nucleus pulposus of a single lumbar disc (L1-L5) with the intent to preserve motion and alleviate pain....
Spinal Stabilization Technologies enrolled the first U.S. patient in its Investigational Device Exemption (IDE) clinical trial for the PerQdisc Nucleus Replacement Device.
The IDE trial will assess the PerQdisc’s ability to replace the nucleus pulposus of a single lumbar disc (L1-L5) with the intent to preserve motion and alleviate pain. Currently, patients with painful lumbar DDD face limited surgical options, with many resorting to spinal fusion, which restricts motion and places increased stresses on adjacent spinal levels. SST’s PerQdisc is a breakthrough alternative that avoids fusion and leaves the surrounding anatomy intact.
To support the trial and accelerate its path toward regulatory approval, SST recently raised $17 million in funding from existing and new investors. This investment will advance the IDE trial and further clinical development initiatives.
“The reduction in pain and overall clinical outcomes observed with PerQdisc during the feasibility trials (over 40 patients treated) have been exceptional,” stated Dr. Pierce Nunley, Principal Investigator and Medical Director of the Spine Institute of Louisiana. “The procedure was notably straightforward, and the recovery period is anticipated to be brief. This technology holds the potential to revolutionize the treatment of patients suffering from discogenic low back pain, providing a straightforward, safe, and minimally invasive option that reduces pain and maintains mobility while restoring functionality.”
Source: Spinal Stabilization Technologies

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







