
 Copy to clipboard
Copy to clipboard 
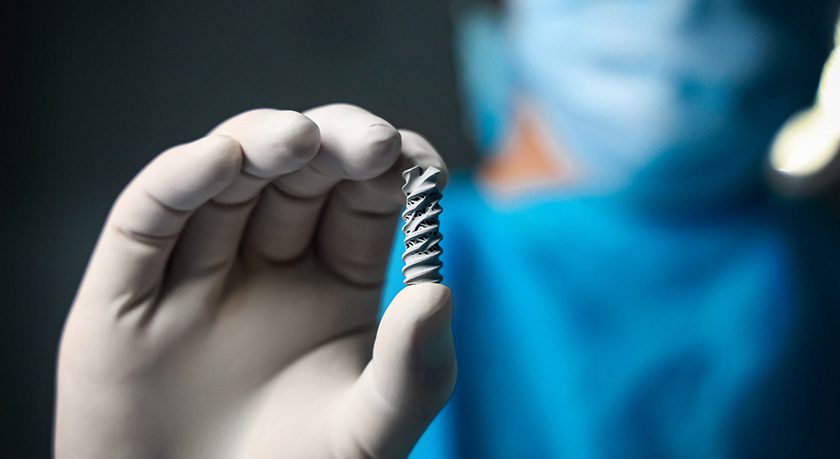
Spinal Simplicity received FDA 510(k) clearance to market the Patriot-SI Posterior Implant System, designed for sacroiliac (SI) joint fusion in combination with the Liberty-SI lateral system. This clearance introduces a hybrid SI joint fusion construct, offering new treatment options for conditions such as sacroiliac disruptions and degenerative sacroiliitis.
When Patriot-SI is implanted, it must be used with a Spinal Simplicity Liberty-SI Lateral System device implanted across the same sacroiliac joint to create a hybrid SI joint fusion construct.
Spinal Simplicity has been granted patent protection for the novel Patriot-SI cannulated and threaded device, which includes an internal lattice structure to promote fusion. The patented cannulated design is reported to be the only posterior SI device that allows continuous control while being delivered over a guidewire.
Patriot-SI is a minimally invasive SI joint fusion implant intended for implantation on a trajectory in line with the joint space. The additively manufactured titanium implant is available in one size, fixating both the sacrum and ilium via external threads. The procedure also involves the lateral insertion of a small titanium Liberty-SI implant, transfixing the SI joint to stabilize and fuse the joint. This hybrid surgical technique supports robust decortication of the bone to prepare the joint for fusion.
CEO Todd Moseley said, “The Patriot SI technology is constructed intentionally as a cannulated implant, designed for repeatable, reproducible techniques, with the implant traveling directly over the guidewire for precision placement in the SI Joint.”
Source: Spinal Simplicity
Spinal Simplicity received FDA 510(k) clearance to market the Patriot-SI Posterior Implant System, designed for sacroiliac (SI) joint fusion in combination with the Liberty-SI lateral system. This clearance introduces a hybrid SI joint fusion construct, offering new treatment options for conditions such as sacroiliac disruptions and degenerative...
Spinal Simplicity received FDA 510(k) clearance to market the Patriot-SI Posterior Implant System, designed for sacroiliac (SI) joint fusion in combination with the Liberty-SI lateral system. This clearance introduces a hybrid SI joint fusion construct, offering new treatment options for conditions such as sacroiliac disruptions and degenerative sacroiliitis.
When Patriot-SI is implanted, it must be used with a Spinal Simplicity Liberty-SI Lateral System device implanted across the same sacroiliac joint to create a hybrid SI joint fusion construct.
Spinal Simplicity has been granted patent protection for the novel Patriot-SI cannulated and threaded device, which includes an internal lattice structure to promote fusion. The patented cannulated design is reported to be the only posterior SI device that allows continuous control while being delivered over a guidewire.
Patriot-SI is a minimally invasive SI joint fusion implant intended for implantation on a trajectory in line with the joint space. The additively manufactured titanium implant is available in one size, fixating both the sacrum and ilium via external threads. The procedure also involves the lateral insertion of a small titanium Liberty-SI implant, transfixing the SI joint to stabilize and fuse the joint. This hybrid surgical technique supports robust decortication of the bone to prepare the joint for fusion.
CEO Todd Moseley said, “The Patriot SI technology is constructed intentionally as a cannulated implant, designed for repeatable, reproducible techniques, with the implant traveling directly over the guidewire for precision placement in the SI Joint.”
Source: Spinal Simplicity

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







