
 Copy to clipboard
Copy to clipboard 
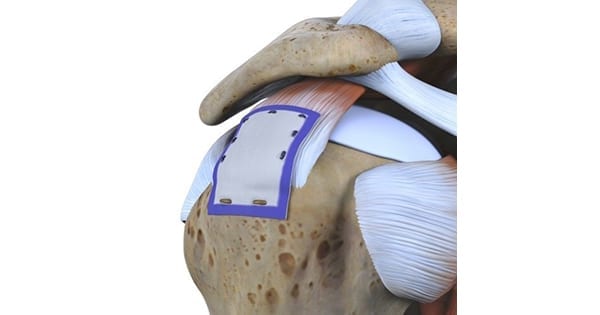
Smith+Nephew received CE Mark approval for the REGENETEN™ Bioinductive Implant. In the repair of rotator cuff injuries, the collagen-based device induces growth of new tendon-like tissue to augment the existing tendon and disrupt disease progression.
REGENETEN is delivered arthroscopically through a small incision over the location of the injury. The implant is about the size of a postage stamp and is completely resorbed within six months.
Since its U.S. introduction, the device has been used in more than 40,000 procedures addressing partial thickness tears to difficult-to-treat large and massive thickness tears. The implant will be available to use on the 310,000+ rotator cuff procedures that take place each year in Europe.
“We are delighted to gain CE Mark for REGENETEN and be able to offer up this ground-breaking technology to our customers in Europe,” said Terry Byca, Senior Director Marketing EMEA, Smith+Nephew. “The U.S. market has demonstrated over the last five years that REGENETEN is changing surgeons’ traditional approach to rotator cuff repair; biological healing is imperative and our Advanced Healing shoulder repair products together with REGENETEN take us into a new era for joint repair.”
Smith+Nephew received CE Mark approval for the REGENETEN™ Bioinductive Implant. In the repair of rotator cuff injuries, the collagen-based device induces growth of new tendon-like tissue to augment the existing tendon and disrupt disease progression.
REGENETEN is delivered arthroscopically through a small incision over the location of the...
Smith+Nephew received CE Mark approval for the REGENETEN™ Bioinductive Implant. In the repair of rotator cuff injuries, the collagen-based device induces growth of new tendon-like tissue to augment the existing tendon and disrupt disease progression.
REGENETEN is delivered arthroscopically through a small incision over the location of the injury. The implant is about the size of a postage stamp and is completely resorbed within six months.
Since its U.S. introduction, the device has been used in more than 40,000 procedures addressing partial thickness tears to difficult-to-treat large and massive thickness tears. The implant will be available to use on the 310,000+ rotator cuff procedures that take place each year in Europe.
“We are delighted to gain CE Mark for REGENETEN and be able to offer up this ground-breaking technology to our customers in Europe,” said Terry Byca, Senior Director Marketing EMEA, Smith+Nephew. “The U.S. market has demonstrated over the last five years that REGENETEN is changing surgeons’ traditional approach to rotator cuff repair; biological healing is imperative and our Advanced Healing shoulder repair products together with REGENETEN take us into a new era for joint repair.”

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







