
 Copy to clipboard
Copy to clipboard 
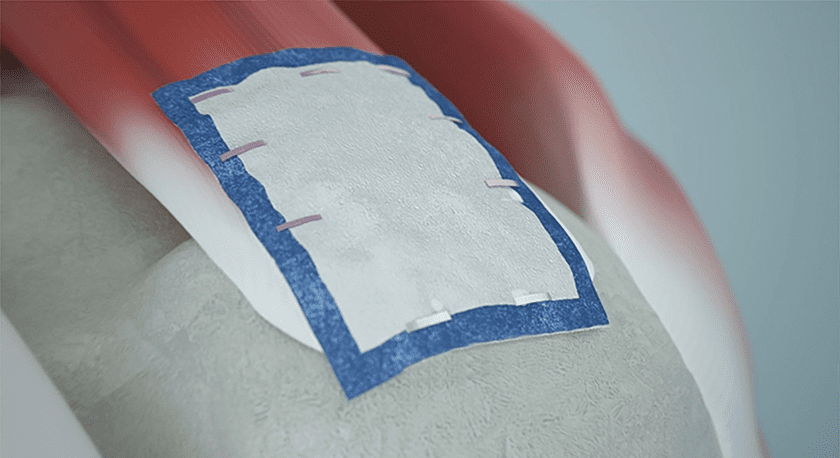
Smith+Nephew shared new evidence and market updates that highlight the clinical performance of its REGENETEN Bioinductive Implant and support its further adoption. Substantial investment into research to expand access and indications have led to these results designed to help surgeons treat more patients with rotator cuff tears and other tendon and extra-articular ligament injuries throughout the body.
AAOS Clinical Practice Guideline (CPG) on the Management of Rotator Cuff Injuries
For the first time, recent updates to the American Academy of Orthopaedic Surgeons (AAOS) CPG on the Management of Rotator Cuff Injuries highlight the value of bioinductive implants in rotator cuff repair, based on independent analysis of studies:
- AAOS CPGs provide evidence-based recommendations for current orthopaedic diagnostic, treatment, and postoperative procedures
- Analyzing RCTs for bioinductive implants, AAOS have issued a Strong Recommendation that ‘The use of bioinductive tendon implants to augment rotator cuff repair, or as an alternative to standard repair, can lead to lower re-tear rates and better patient reported outcomes.’
Randomized Controlled Trial (RCT) Results
Two-year results of the MALLAMANGUITO RCT demonstrated a sustained 65% relative reduction in re-tear rates when using the REGENETEN Bioinductive Implant to augment repair of full-thickness rotator cuff tears:
- Two-year re-tear rates were 12.3% (7/57) with the REGENETEN Implant vs. 35.1% (20/57) with standard repair (p=0.004).
- These results build on previously reported 1-year results which have already made a significant impact in supporting patient access and adoption.
Extra-articular Ligaments
For the first time, Smith+Nephew is able to market the REGENETEN Bioinductive Implant for extra-articular ligament injuries in the US. This new indication expansion offers opportunities to reach more patients with soft tissue injuries throughout the body. It will initially focus on hip capsule repair with abundant opportunities for future expansions in other extra-articular ligament repairs.
With more than 150,000 procedures completed globally since its introduction in 2014, the REGENETEN Bioinductive Implant has had a transformative impact, offering a better solution for the more than 1 million people having tendon or extra-articular ligament repair each year. The collagen-based implant supports the body’s natural healing response to facilitate the formation of new tissue to biologically augment repairs and support natural healing processes.
“These significant updates for the REGENETEN Implant are the culmination of many years of hard work from both the Smith+Nephew team and surgeon community,” said Scott Schaffner, President Global Sports Medicine at Smith+Nephew. “We are excited about its future potential to treat even more patients who require surgical repair of soft tissue injuries every year.”
Source: Smith+Nephew
Smith+Nephew shared new evidence and market updates that highlight the clinical performance of its REGENETEN Bioinductive Implant and support its further adoption. Substantial investment into research to expand access and indications have led to these results designed to help surgeons treat more patients with rotator cuff tears and other tendon...
Smith+Nephew shared new evidence and market updates that highlight the clinical performance of its REGENETEN Bioinductive Implant and support its further adoption. Substantial investment into research to expand access and indications have led to these results designed to help surgeons treat more patients with rotator cuff tears and other tendon and extra-articular ligament injuries throughout the body.
AAOS Clinical Practice Guideline (CPG) on the Management of Rotator Cuff Injuries
For the first time, recent updates to the American Academy of Orthopaedic Surgeons (AAOS) CPG on the Management of Rotator Cuff Injuries highlight the value of bioinductive implants in rotator cuff repair, based on independent analysis of studies:
- AAOS CPGs provide evidence-based recommendations for current orthopaedic diagnostic, treatment, and postoperative procedures
- Analyzing RCTs for bioinductive implants, AAOS have issued a Strong Recommendation that ‘The use of bioinductive tendon implants to augment rotator cuff repair, or as an alternative to standard repair, can lead to lower re-tear rates and better patient reported outcomes.’
Randomized Controlled Trial (RCT) Results
Two-year results of the MALLAMANGUITO RCT demonstrated a sustained 65% relative reduction in re-tear rates when using the REGENETEN Bioinductive Implant to augment repair of full-thickness rotator cuff tears:
- Two-year re-tear rates were 12.3% (7/57) with the REGENETEN Implant vs. 35.1% (20/57) with standard repair (p=0.004).
- These results build on previously reported 1-year results which have already made a significant impact in supporting patient access and adoption.
Extra-articular Ligaments
For the first time, Smith+Nephew is able to market the REGENETEN Bioinductive Implant for extra-articular ligament injuries in the US. This new indication expansion offers opportunities to reach more patients with soft tissue injuries throughout the body. It will initially focus on hip capsule repair with abundant opportunities for future expansions in other extra-articular ligament repairs.
With more than 150,000 procedures completed globally since its introduction in 2014, the REGENETEN Bioinductive Implant has had a transformative impact, offering a better solution for the more than 1 million people having tendon or extra-articular ligament repair each year. The collagen-based implant supports the body’s natural healing response to facilitate the formation of new tissue to biologically augment repairs and support natural healing processes.
“These significant updates for the REGENETEN Implant are the culmination of many years of hard work from both the Smith+Nephew team and surgeon community,” said Scott Schaffner, President Global Sports Medicine at Smith+Nephew. “We are excited about its future potential to treat even more patients who require surgical repair of soft tissue injuries every year.”
Source: Smith+Nephew

You’ve reached your limit.
We’re glad you’re finding value in our content — and we’d love for you to keep going.
Subscribe now for unlimited access to orthopedic business intelligence.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







