
 Copy to clipboard
Copy to clipboard 
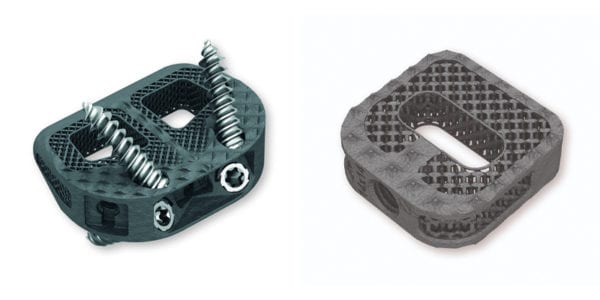
Signus Medizintechnik received CE Mark approval for the BIG® ST ALIF Cage, an addition to the company’s Structural Titanum portfolio of 3D-printed cages.
The device can be inserted either from anterior or minimally invasive retro/transperitoneal approaches, inserted in 45° oblique or 90° lateral techniques. Implanted anteriorly, BIG ST can be used as a standalone fusion device. The flattened front edge and smooth lateral surfaces, combined with the self distracting design, ensure a reduced preparation and protection of nerve structures during insertion.
An open macroporous structure resembles natural cancellous architecture and enables both on- and ingrowth of bone that can be supported by filling the implant with KAINOS® Inject bone graft substitute. Increased roughness in conjunction with the SIGNUS toothed cage design reduces the risk of implant migration, and a large contact area with the vertebral body reduces the risk of subsidence.
Earlier this year, the company received CE Mark approval for the JASPIS® ST cervical cage. The device design originates from the RABEA cage, which received the world’s first CE Mark approval for a plane-parallel fusion cage in 1996.
JASPIS’ titanium grid structure imitates the architecture of natural cancellous bone to optimize intercorporeal fusion, while the open design permits the cage to be packed with natural or synthetic bone graft substitute such as KAINOS Inject.
Signus Medizintechnik received CE Mark approval for the BIG® ST ALIF Cage, an addition to the company's Structural Titanum portfolio of 3D-printed cages.
The device can be inserted either from anterior or minimally invasive retro/transperitoneal approaches, inserted in 45° oblique or 90° lateral techniques. Implanted anteriorly, BIG ST can be...
Signus Medizintechnik received CE Mark approval for the BIG® ST ALIF Cage, an addition to the company’s Structural Titanum portfolio of 3D-printed cages.
The device can be inserted either from anterior or minimally invasive retro/transperitoneal approaches, inserted in 45° oblique or 90° lateral techniques. Implanted anteriorly, BIG ST can be used as a standalone fusion device. The flattened front edge and smooth lateral surfaces, combined with the self distracting design, ensure a reduced preparation and protection of nerve structures during insertion.
An open macroporous structure resembles natural cancellous architecture and enables both on- and ingrowth of bone that can be supported by filling the implant with KAINOS® Inject bone graft substitute. Increased roughness in conjunction with the SIGNUS toothed cage design reduces the risk of implant migration, and a large contact area with the vertebral body reduces the risk of subsidence.
Earlier this year, the company received CE Mark approval for the JASPIS® ST cervical cage. The device design originates from the RABEA cage, which received the world’s first CE Mark approval for a plane-parallel fusion cage in 1996.
JASPIS’ titanium grid structure imitates the architecture of natural cancellous bone to optimize intercorporeal fusion, while the open design permits the cage to be packed with natural or synthetic bone graft substitute such as KAINOS Inject.

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







