
 Copy to clipboard
Copy to clipboard 
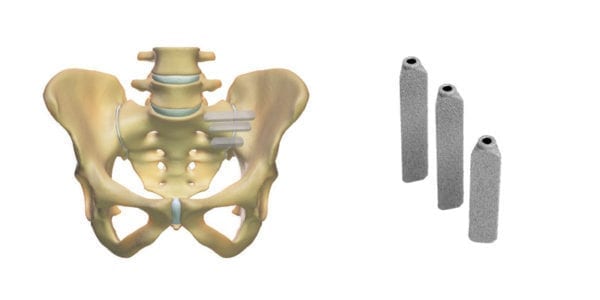
UnitedHealthcare changed its minimally invasive sacroiliac joint fusion policy from covering all devices to exclusively covering SI-BONE’s triangular iFuse Implants, effective October 1, 2021.
UnitedHealthcare has adopted InterQual Clinical Criteria for Sacroiliac Joint Interventions, updating its policies to cover minimally invasive SI joint fusion using a Titanium Triangular Implant (i.e., iFuse Implant System).
UnitedHealthcare is the largest commercial payor in the U.S., with over 45 million members, and joins Anthem, Humana and many Blue Cross Blue Shield Association health plans in requiring use of the triangular iFuse Implant System for minimally invasive SI joint fusion. With this updated policy, UnitedHealthcare joins more than 35 other health plans that collectively cover over 160 million insured, requiring the use of the iFuse Implant System for minimally invasive SI joint fusion. Nearly all other U.S. insurers now cover the procedure, generally.
The revised policy provides coverage for minimally invasive sacroiliac joint fusion for the treatment of painful degenerative SI joint disease, provided certain criteria are met. Until now, UnitedHealthcare has generally covered minimally invasive SI joint fusion procedures, but has not distinguished the implant type used. T
Source: SI-BONE, Inc.
UnitedHealthcare changed its minimally invasive sacroiliac joint fusion policy from covering all devices to exclusively covering SI-BONE’s triangular iFuse Implants, effective October 1, 2021.
UnitedHealthcare has adopted InterQual Clinical Criteria for Sacroiliac Joint Interventions, updating its policies to cover minimally invasive SI joint...
UnitedHealthcare changed its minimally invasive sacroiliac joint fusion policy from covering all devices to exclusively covering SI-BONE’s triangular iFuse Implants, effective October 1, 2021.
UnitedHealthcare has adopted InterQual Clinical Criteria for Sacroiliac Joint Interventions, updating its policies to cover minimally invasive SI joint fusion using a Titanium Triangular Implant (i.e., iFuse Implant System).
UnitedHealthcare is the largest commercial payor in the U.S., with over 45 million members, and joins Anthem, Humana and many Blue Cross Blue Shield Association health plans in requiring use of the triangular iFuse Implant System for minimally invasive SI joint fusion. With this updated policy, UnitedHealthcare joins more than 35 other health plans that collectively cover over 160 million insured, requiring the use of the iFuse Implant System for minimally invasive SI joint fusion. Nearly all other U.S. insurers now cover the procedure, generally.
The revised policy provides coverage for minimally invasive sacroiliac joint fusion for the treatment of painful degenerative SI joint disease, provided certain criteria are met. Until now, UnitedHealthcare has generally covered minimally invasive SI joint fusion procedures, but has not distinguished the implant type used. T
Source: SI-BONE, Inc.

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







