
 Copy to clipboard
Copy to clipboard 
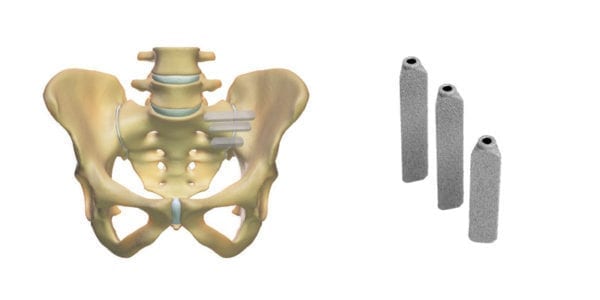
SI-BONE announced that Priority Health Michigan became the 36th U.S. payor with an exclusive, positive iFuse coverage policy. Prior to its November 2020 update, the Priority Health policy covered sacroiliac (SI) joint fusion without a requirement that any particular implant be used. This change provides coverage for minimally invasive (MIS) SI joint fusion for the treatment of lower back pain with a diagnosis of sacroiliac joint disruption or degenerative sacroiliitis. MIS SI joint fusion will remain experimental and/or investigational for all other systems and approaches, including cylindrical threaded implants.
Priority Health is the second-largest health plan in the state of Michigan, with more than 780,000 covered lives. Priority Health has enrollees in commercial, Medicare Advantage and Medicaid health plans that it operates.
SI-BONE also noted that the Blue Cross Blue Shield Association (BCBSA) Evidence Street® Opinion on Diagnosis and Treatment of Sacroiliac Joint Pain was updated in December to reflect recent evidence for triangular implants (e.g., iFuse) as well as for all other types of implants used in SI joint fusion procedures.
The updated BCBSA Opinion states that for individuals with common disorders affecting the sacroiliac joint who are treated with sacroiliac fusion/fixation with triangular implants, the evidence is sufficient to determine that the technology results in a meaningful improvement in the net health outcome. By contrast, the BCBSA Opinion states that the evidence is insufficient to determine the net health outcome for patients treated with cylindrical threaded implants, therapeutic corticosteroid injections, or with radiofrequency ablation.
While multiple companies have developed and market devices for sacroiliac fusion, SI-BONE continues to rack up coverage for its iFuse product family.
“Priority Health’s decision to limit its coverage policy for its members to minimally invasive SI joint fusion procedures exclusively using iFuse was supported by more than 85 peer-reviewed articles reviewing iFuse patient outcomes, including randomized controlled trials and other independent studies, which is in line with the Blue Cross Blue Shield Assocation Opinion published this month.” – Jeffrey Zigler, Vice President of Market Access and Reimbursement at SI-BONE
SI-BONE announced that Priority Health Michigan became the 36th U.S. payor with an exclusive, positive iFuse coverage policy. Prior to its November 2020 update, the Priority Health policy covered sacroiliac (SI) joint fusion without a requirement that any particular implant be used. This change provides coverage for minimally invasive (MIS)...
SI-BONE announced that Priority Health Michigan became the 36th U.S. payor with an exclusive, positive iFuse coverage policy. Prior to its November 2020 update, the Priority Health policy covered sacroiliac (SI) joint fusion without a requirement that any particular implant be used. This change provides coverage for minimally invasive (MIS) SI joint fusion for the treatment of lower back pain with a diagnosis of sacroiliac joint disruption or degenerative sacroiliitis. MIS SI joint fusion will remain experimental and/or investigational for all other systems and approaches, including cylindrical threaded implants.
Priority Health is the second-largest health plan in the state of Michigan, with more than 780,000 covered lives. Priority Health has enrollees in commercial, Medicare Advantage and Medicaid health plans that it operates.
SI-BONE also noted that the Blue Cross Blue Shield Association (BCBSA) Evidence Street® Opinion on Diagnosis and Treatment of Sacroiliac Joint Pain was updated in December to reflect recent evidence for triangular implants (e.g., iFuse) as well as for all other types of implants used in SI joint fusion procedures.
The updated BCBSA Opinion states that for individuals with common disorders affecting the sacroiliac joint who are treated with sacroiliac fusion/fixation with triangular implants, the evidence is sufficient to determine that the technology results in a meaningful improvement in the net health outcome. By contrast, the BCBSA Opinion states that the evidence is insufficient to determine the net health outcome for patients treated with cylindrical threaded implants, therapeutic corticosteroid injections, or with radiofrequency ablation.
While multiple companies have developed and market devices for sacroiliac fusion, SI-BONE continues to rack up coverage for its iFuse product family.
“Priority Health’s decision to limit its coverage policy for its members to minimally invasive SI joint fusion procedures exclusively using iFuse was supported by more than 85 peer-reviewed articles reviewing iFuse patient outcomes, including randomized controlled trials and other independent studies, which is in line with the Blue Cross Blue Shield Assocation Opinion published this month.” – Jeffrey Zigler, Vice President of Market Access and Reimbursement at SI-BONE

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







