
 Copy to clipboard
Copy to clipboard 
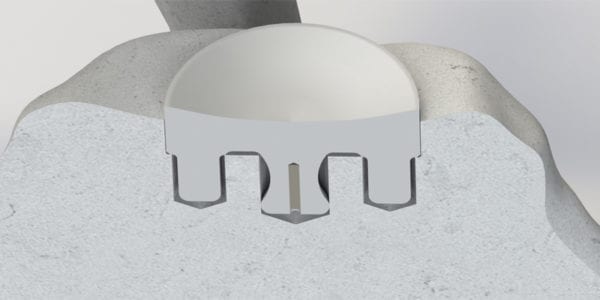
Shoulder Innovations was granted FDA 510(k) clearance to market InSet Plus™ augmented glenoids for the InSet™ Total Shoulder.
The new glenoids feature an angled articular surface and are available in both 5° and 10° variants with multiple diameters to treat significant disability in degenerative, rheumatoid and traumatic disease of the glenohumeral joint and avascular necrosis of the humeral head.
Studies have shown that the InSet Shoulder demonstrated an 87% reduction in implant micro-motion vs. conventional glenoid designs, with no complications, no cases of glenoid implant loosening and no revisions performed in the series at a mean 8.7-year follow-up.
Don Running, Vice President, Research and Development for Shoulder Innovations, said, “The key to the long-term clinical success of the InSet glenoid has been both the unique flat back design and novel bone pocket created in the fossa to allow for a secure fixation that is ‘set in’ the subchondral bone. With the new InSet Plus design, surgeons have the ability to create optimal pocket depths on more eroded glenoid faces while still providing significant version correction. In addition, due to the circular nature of the implant design, surgeons are able to dial the augmentation to provide stability in multiple positions.”
Shoulder Innovations was granted FDA 510(k) clearance to market InSet Plus™ augmented glenoids for the InSet™ Total Shoulder.
The new glenoids feature an angled articular surface and are available in both 5° and 10° variants with multiple diameters to treat significant disability in degenerative, rheumatoid and traumatic disease of the...
Shoulder Innovations was granted FDA 510(k) clearance to market InSet Plus™ augmented glenoids for the InSet™ Total Shoulder.
The new glenoids feature an angled articular surface and are available in both 5° and 10° variants with multiple diameters to treat significant disability in degenerative, rheumatoid and traumatic disease of the glenohumeral joint and avascular necrosis of the humeral head.
Studies have shown that the InSet Shoulder demonstrated an 87% reduction in implant micro-motion vs. conventional glenoid designs, with no complications, no cases of glenoid implant loosening and no revisions performed in the series at a mean 8.7-year follow-up.
Don Running, Vice President, Research and Development for Shoulder Innovations, said, “The key to the long-term clinical success of the InSet glenoid has been both the unique flat back design and novel bone pocket created in the fossa to allow for a secure fixation that is ‘set in’ the subchondral bone. With the new InSet Plus design, surgeons have the ability to create optimal pocket depths on more eroded glenoid faces while still providing significant version correction. In addition, due to the circular nature of the implant design, surgeons are able to dial the augmentation to provide stability in multiple positions.”

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







