
 Copy to clipboard
Copy to clipboard 
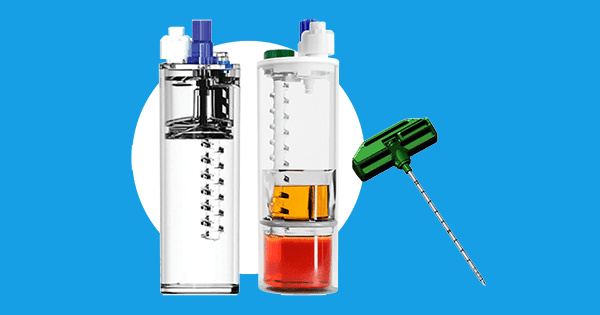
Royal Biologics announced FDA 510(k) clearance and U.S. launch of MAXX™-BMC. MAXX-BMC is a bone marrow aspirate concentration system that provides users with an optimal sample of concentrated bone marrow aspirate from a patient at point of care. Royal’s “Lead Screw” technology in their Maxx concentration device allows users to develop a customized sample for use during orthopedic and sports medicine surgical procedures. MAXX-BMC is the latest addition featured in Royal Biologics’ portfolio of Advanced Cellular Technologies & Enhanced Autologous Healing systems.
Lead Screw technology allows clinicians to pinpoint precise and accurate areas of the “Buffy Coat” fraction (growth factor layer) of bone marrow aspirate in a matter of seconds post-concentration. Maxx-BMC provides up to two concentration cycles in eight minutes.
Royal Biologics’ Enhanced Autologous Healing and Advanced Cellular Portfolio now comprises five systems: FIBRINET™ (Platelet Rich Fibrin Matrix), OSTEO™-SPIN, MAXX™-Cell (bone marrow harvester), MAXX™-PRP (Platelet Rich Plasma) and MAXX-BMC (Bone Marrow Aspirate Concentrate).
Source: Royal Biologics
Royal Biologics announced FDA 510(k) clearance and U.S. launch of MAXX™-BMC. MAXX-BMC is a bone marrow aspirate concentration system that provides users with an optimal sample of concentrated bone marrow aspirate from a patient at point of care. Royal's "Lead Screw" technology in their Maxx concentration device allows users to develop a...
Royal Biologics announced FDA 510(k) clearance and U.S. launch of MAXX™-BMC. MAXX-BMC is a bone marrow aspirate concentration system that provides users with an optimal sample of concentrated bone marrow aspirate from a patient at point of care. Royal’s “Lead Screw” technology in their Maxx concentration device allows users to develop a customized sample for use during orthopedic and sports medicine surgical procedures. MAXX-BMC is the latest addition featured in Royal Biologics’ portfolio of Advanced Cellular Technologies & Enhanced Autologous Healing systems.
Lead Screw technology allows clinicians to pinpoint precise and accurate areas of the “Buffy Coat” fraction (growth factor layer) of bone marrow aspirate in a matter of seconds post-concentration. Maxx-BMC provides up to two concentration cycles in eight minutes.
Royal Biologics’ Enhanced Autologous Healing and Advanced Cellular Portfolio now comprises five systems: FIBRINET™ (Platelet Rich Fibrin Matrix), OSTEO™-SPIN, MAXX™-Cell (bone marrow harvester), MAXX™-PRP (Platelet Rich Plasma) and MAXX-BMC (Bone Marrow Aspirate Concentrate).
Source: Royal Biologics

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







