
 Copy to clipboard
Copy to clipboard 
Rivanna Medical received funding of $30.5 million over 39 months from early execution of an option by the Biomedical Advanced Research and Development Authority (BARDA), part of the Administration for Strategic Preparedness and Response within the U.S. Department of Health and Human Services.
The influx will support further development of the Accuro XV for comprehensive point-of-care musculoskeletal diagnostics, as well as submission of an application for FDA clearance.
The contract includes options for additional funding of up to $56.4 million to support the development leading to FDA 510(k) clearance for CADe/x artificial intelligence (AI)-enabled fracture detection. This second round of funding follows an $11.6 million base contract executed in 2021 for the proof-of-concept product development and market assessment of Accuro XV. The project aims to develop an FDA-cleared and clinically proven product to expedite triage of low-acuity extremity injuries in emergency medicine, with a particular focus on mass-casualty blast trauma incidents.
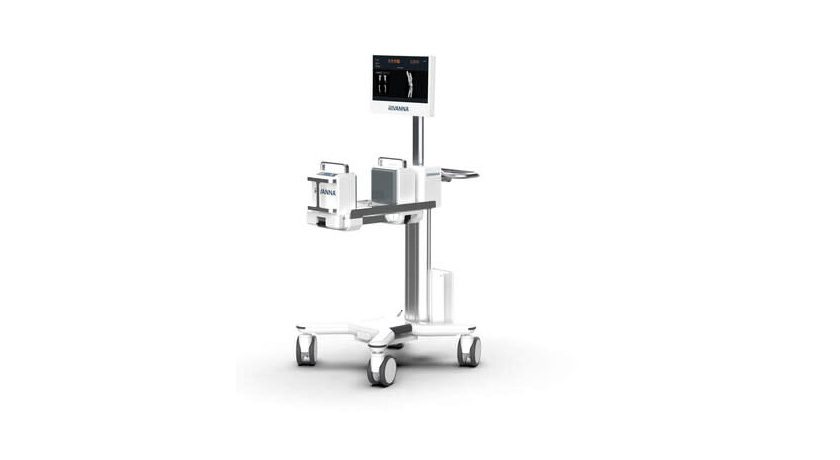
Accuro XV integrates ultrasound-based bone and 3D soft-tissue imaging technology with AI-enabled software, offering advantages over traditional x-ray methods. The system is designed to enable rapid automated detection and precise volumetric assessment of skeletal fractures and soft tissue injuries without exposing patients to harmful radiation or requiring extensive user training. Clinicians may make informed decisions regarding the necessity of x-ray imaging, effectively reducing nonessential radiography. Additionally, Accuro XV may address a current limitation in emergency departments by swiftly determining soft tissue injuries during the acute phase; this would ensure accurate treatment decisions and prompt discharge of individuals who do not require further intervention.
Led by Will Mauldin, PhD, co-founder and CEO, Rivanna is elevating global standards of care through the development and commercialization of imaging-based medical technologies, including BoneVision Multi-Probe Multi-Angle Image Acquisition, BoneEnhance Multi-Frequency Image Reconstruction and SpineNav3D AI-Enabled Spine Recognition.
Source: Rivanna Medical
Rivanna Medical received funding of $30.5 million over 39 months from early execution of an option by the Biomedical Advanced Research and Development Authority (BARDA), part of the Administration for Strategic Preparedness and Response within the U.S. Department of Health and Human Services.
The influx will support further development of the...
Rivanna Medical received funding of $30.5 million over 39 months from early execution of an option by the Biomedical Advanced Research and Development Authority (BARDA), part of the Administration for Strategic Preparedness and Response within the U.S. Department of Health and Human Services.
The influx will support further development of the Accuro XV for comprehensive point-of-care musculoskeletal diagnostics, as well as submission of an application for FDA clearance.
The contract includes options for additional funding of up to $56.4 million to support the development leading to FDA 510(k) clearance for CADe/x artificial intelligence (AI)-enabled fracture detection. This second round of funding follows an $11.6 million base contract executed in 2021 for the proof-of-concept product development and market assessment of Accuro XV. The project aims to develop an FDA-cleared and clinically proven product to expedite triage of low-acuity extremity injuries in emergency medicine, with a particular focus on mass-casualty blast trauma incidents.
Accuro XV integrates ultrasound-based bone and 3D soft-tissue imaging technology with AI-enabled software, offering advantages over traditional x-ray methods. The system is designed to enable rapid automated detection and precise volumetric assessment of skeletal fractures and soft tissue injuries without exposing patients to harmful radiation or requiring extensive user training. Clinicians may make informed decisions regarding the necessity of x-ray imaging, effectively reducing nonessential radiography. Additionally, Accuro XV may address a current limitation in emergency departments by swiftly determining soft tissue injuries during the acute phase; this would ensure accurate treatment decisions and prompt discharge of individuals who do not require further intervention.
Led by Will Mauldin, PhD, co-founder and CEO, Rivanna is elevating global standards of care through the development and commercialization of imaging-based medical technologies, including BoneVision Multi-Probe Multi-Angle Image Acquisition, BoneEnhance Multi-Frequency Image Reconstruction and SpineNav3D AI-Enabled Spine Recognition.
Source: Rivanna Medical

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







