
 Copy to clipboard
Copy to clipboard 
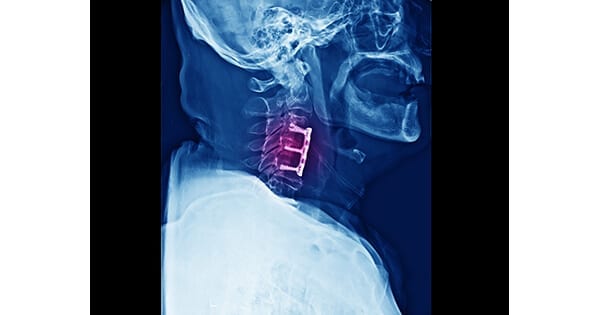
ReVivo Medical was awarded its 11th U.S. patent, pertaining to anterior cervical plate and interbody cage technology. The company also holds two patents overseas.
ReVivo Medical is developing elastic micro-motion implantable spinal devices. Results from an efficacy study demonstrated that loadsharing through elastic micro-motion accelerates bone formation and interbody fusion.
The company intends to begin a clinical study in early 2021 to support an application for FDA 510(k) clearance of their anterior cervical plates and interbody cages.
“Our goal at ReVivo Medical is to invent and bring to market medical devices that will improve patient outcomes and reduce costs.” says Gary Mittleman, President and CEO. “Our defensible competitive position is only as good as the patents that protect our designs. Eight years of hard work has culminated in an array of patents that we believe will provide broad protection for a multi-billion-dollar market.”
“Our cervical plate and cage implants are designed to improve bone formation and achieve a superior rate and quality of fusion as compared to the commonly used state of the art devices in use today,” said Eric Ledet, Ph.D., Chief Science Officer. “Additionally, the designs of our implants incorporate unique features to make it easier for the surgeon to use.”
ReVivo Medical was awarded its 11th U.S. patent, pertaining to anterior cervical plate and interbody cage technology. The company also holds two patents overseas.
ReVivo Medical is developing elastic micro-motion implantable spinal devices. Results from an efficacy study demonstrated that loadsharing through elastic micro-motion...
ReVivo Medical was awarded its 11th U.S. patent, pertaining to anterior cervical plate and interbody cage technology. The company also holds two patents overseas.
ReVivo Medical is developing elastic micro-motion implantable spinal devices. Results from an efficacy study demonstrated that loadsharing through elastic micro-motion accelerates bone formation and interbody fusion.
The company intends to begin a clinical study in early 2021 to support an application for FDA 510(k) clearance of their anterior cervical plates and interbody cages.
“Our goal at ReVivo Medical is to invent and bring to market medical devices that will improve patient outcomes and reduce costs.” says Gary Mittleman, President and CEO. “Our defensible competitive position is only as good as the patents that protect our designs. Eight years of hard work has culminated in an array of patents that we believe will provide broad protection for a multi-billion-dollar market.”
“Our cervical plate and cage implants are designed to improve bone formation and achieve a superior rate and quality of fusion as compared to the commonly used state of the art devices in use today,” said Eric Ledet, Ph.D., Chief Science Officer. “Additionally, the designs of our implants incorporate unique features to make it easier for the surgeon to use.”

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







