
 Copy to clipboard
Copy to clipboard 
ReVivo Medical completed its most recent equity offering.
“Since last fall we have now raised $2 million which will allow us to complete the planned 50 surgical procedures of our clinical trial,” said Gary Mittleman, President and CEO. “Raising funds is never an easy task and we are grateful to the support of the 57 angel investors who have helped to fund our development activities over the last 6 years.”
“Attaining FDA clearance is the final step before offering a commercial product and this trial is our gateway to achieving this goal,” said Eric Ledet, Chief Science Officer. “We expect to begin enrolling patients for our study shortly.”
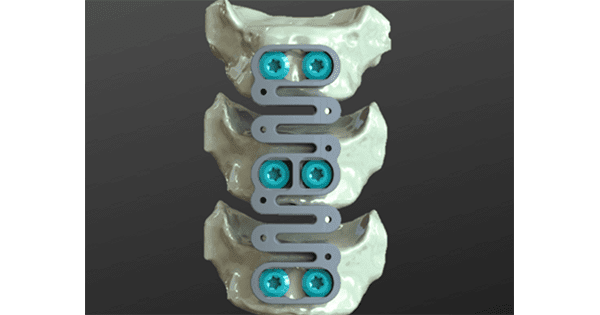
Study participants will receive ReVivo Medical’s next-generation design anterior cervical plate and interbody cages used in anterior cervical discectomy and fusion procedures.
Source: ReVivo Medical
ReVivo Medical completed its most recent equity offering.
“Since last fall we have now raised $2 million which will allow us to complete the planned 50 surgical procedures of our clinical trial,” said Gary Mittleman, President and CEO. “Raising funds is never an easy task and we are grateful to the support of the 57 angel investors who...
ReVivo Medical completed its most recent equity offering.
“Since last fall we have now raised $2 million which will allow us to complete the planned 50 surgical procedures of our clinical trial,” said Gary Mittleman, President and CEO. “Raising funds is never an easy task and we are grateful to the support of the 57 angel investors who have helped to fund our development activities over the last 6 years.”
“Attaining FDA clearance is the final step before offering a commercial product and this trial is our gateway to achieving this goal,” said Eric Ledet, Chief Science Officer. “We expect to begin enrolling patients for our study shortly.”
Study participants will receive ReVivo Medical’s next-generation design anterior cervical plate and interbody cages used in anterior cervical discectomy and fusion procedures.
Source: ReVivo Medical

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







