
 Copy to clipboard
Copy to clipboard 
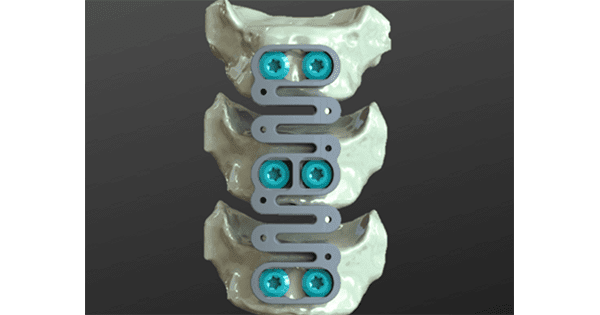
ReVivo Medical closed on a private equity offering, raising over $550,000. The company is developing elastic micro-motion implantable spinal devices. Results from an efficacy study demonstrated that loadsharing through elastic micro-motion may accelerate bone formation and interbody fusion.
ReVivo Medical intends to begin an FDA-approved 50 patient clinical study in 2Q21 to support its application for clearance of anterior cervical plates and interbody cages for commercial use. ReVivo intends to raise additional funds as the trial progresses.
Gary Mittleman, President and CEO, said, “Obtaining FDA market clearance for our flagship devices is our next hurdle. Although less than we were targeting to raise, this financing will enable us to kick off the early stages of a clinical trial, essential for this clearance.”
“Our cervical plate and cage implants are designed to improve bone formation and achieve a superior rate and quality of fusion as compared to the commonly used state of the art devices in use today,” said Eric Ledet, Ph.D., Chief Science Officer.
ReVivo Medical closed on a private equity offering, raising over $550,000. The company is developing elastic micro-motion implantable spinal devices. Results from an efficacy study demonstrated that loadsharing through elastic micro-motion may accelerate bone formation and interbody fusion.
ReVivo Medical intends to begin an FDA-approved...
ReVivo Medical closed on a private equity offering, raising over $550,000. The company is developing elastic micro-motion implantable spinal devices. Results from an efficacy study demonstrated that loadsharing through elastic micro-motion may accelerate bone formation and interbody fusion.
ReVivo Medical intends to begin an FDA-approved 50 patient clinical study in 2Q21 to support its application for clearance of anterior cervical plates and interbody cages for commercial use. ReVivo intends to raise additional funds as the trial progresses.
Gary Mittleman, President and CEO, said, “Obtaining FDA market clearance for our flagship devices is our next hurdle. Although less than we were targeting to raise, this financing will enable us to kick off the early stages of a clinical trial, essential for this clearance.”
“Our cervical plate and cage implants are designed to improve bone formation and achieve a superior rate and quality of fusion as compared to the commonly used state of the art devices in use today,” said Eric Ledet, Ph.D., Chief Science Officer.

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







