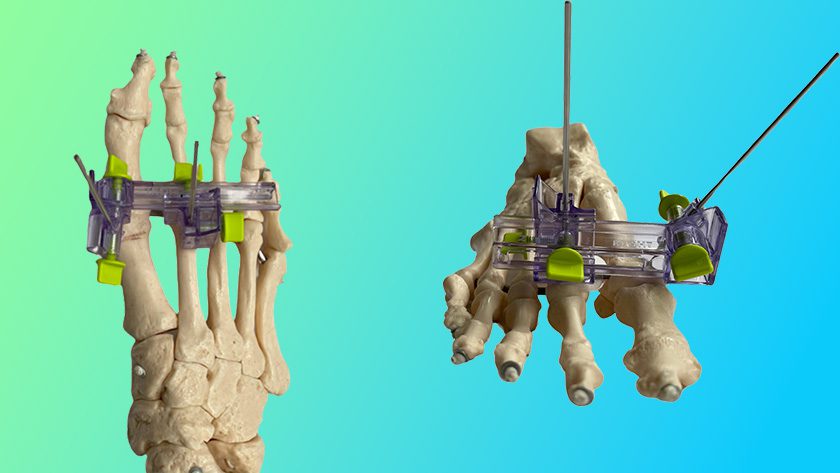
Renovis Surgical received FDA 510(k) clearance to market Tesera® porous titanium posterior lumbar interbody fusion systems.
All Tesera implants are made using additive manufacturing techniques and Tesera Trabecular Technology®, yielding a highly porous structure to support bone attachment.
This is Renovis’ fifth product group featuring Tesera. Earlier FDA-cleared products include Tesera SA for standalone anterior fusion, the Tesera acetabular system for total hip reconstruction, the original Tesera PLIF line and Tesera SC for standalone anterior cervical fusion.
Sources: Renovis Surgical Technologies, Inc.; ORTHOWORLD Inc.
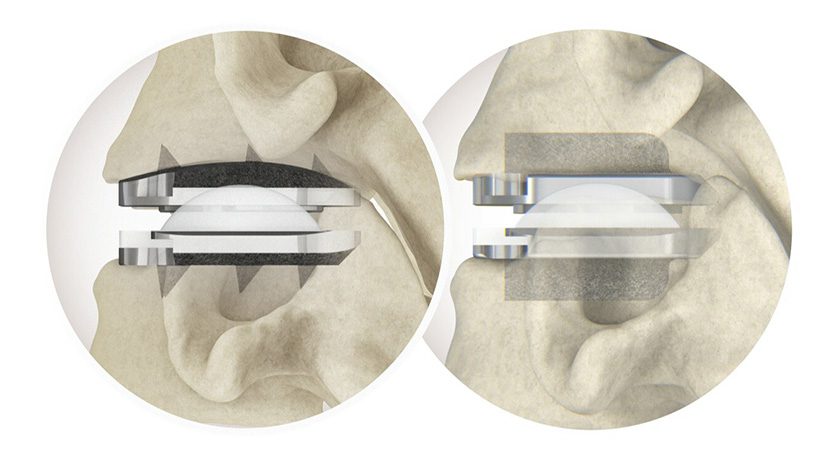
Renovis Surgical received FDA 510(k) clearance to market Tesera® porous titanium posterior lumbar interbody fusion systems.
All Tesera implants are made using additive manufacturing techniques and Tesera Trabecular Technology®, yielding a highly porous structure to support bone attachment.
This is Renovis' fifth product group featuring...
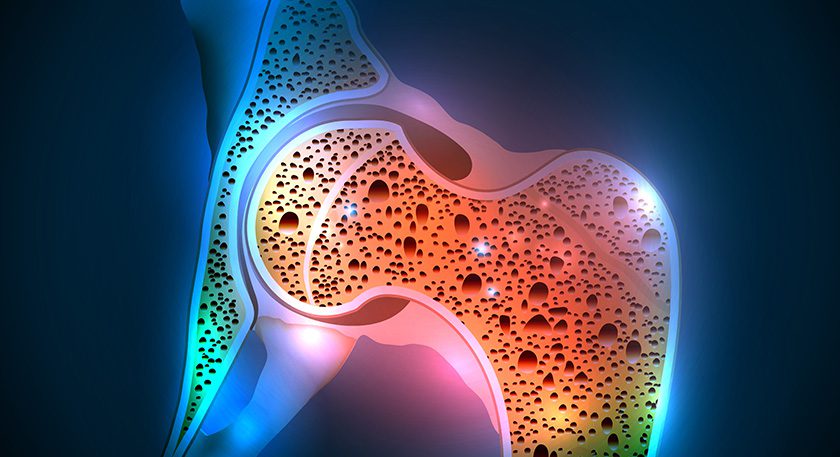
Renovis Surgical received FDA 510(k) clearance to market Tesera® porous titanium posterior lumbar interbody fusion systems.
All Tesera implants are made using additive manufacturing techniques and Tesera Trabecular Technology®, yielding a highly porous structure to support bone attachment.
This is Renovis’ fifth product group featuring Tesera. Earlier FDA-cleared products include Tesera SA for standalone anterior fusion, the Tesera acetabular system for total hip reconstruction, the original Tesera PLIF line and Tesera SC for standalone anterior cervical fusion.
Sources: Renovis Surgical Technologies, Inc.; ORTHOWORLD Inc.


You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.