
 Copy to clipboard
Copy to clipboard 
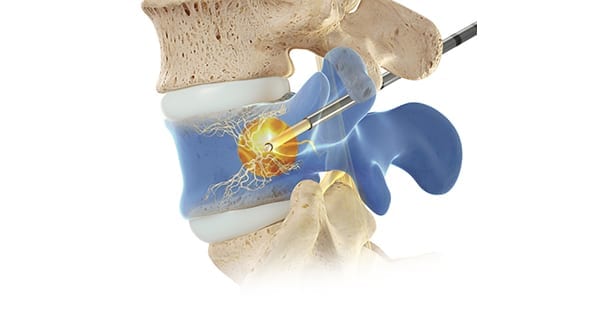
Long-term study data indicate that Relievant Medsystems’ Intracept procedure showed durability of improvements in pain and function beyond five years in the treatment of chronic low back pain (CLBP).
The Level I SMART trial is a single-arm, open-label, prospective follow-up of 100 U.S. patients (85% retention) who were successfully treated with basivertebral nerve ablation in the randomized study.
Significant differences were demonstrated at a minimum of five years post-procedure, including the following:
- Nearly half of patients reported 75% or greater reduction in pain; more than a third reported complete pain resolution
- 73% reduction in active opioid use
- High patient satisfaction with the procedure
- 79% indicated they would have the procedure again for the same condition
- 65% had resumed the level of activity they enjoyed prior to low back pain
“Following publication of two Level I Randomized Control Trials, the SMART study and the INTRACEPT study, we are pleased to see such a meaningful degree of clinical improvement being maintained more than five years after patients received the Intracept procedure,” said Art Taylor, President & CEO of Relievant Medsystems. “This unparalleled level of clinical evidence demonstrating that Intracept is a highly-effective and durable therapy with a very favorable safety profile, is strongly compelling for physicians, payers, and most of all, patients suffering from vertebrogenic CLBP.”
Long-term study data indicate that Relievant Medsystems' Intracept procedure showed durability of improvements in pain and function beyond five years in the treatment of chronic low back pain (CLBP).
The Level I SMART trial is a single-arm, open-label, prospective follow-up of 100 U.S. patients (85% retention) who were successfully treated...
Long-term study data indicate that Relievant Medsystems’ Intracept procedure showed durability of improvements in pain and function beyond five years in the treatment of chronic low back pain (CLBP).
The Level I SMART trial is a single-arm, open-label, prospective follow-up of 100 U.S. patients (85% retention) who were successfully treated with basivertebral nerve ablation in the randomized study.
Significant differences were demonstrated at a minimum of five years post-procedure, including the following:
- Nearly half of patients reported 75% or greater reduction in pain; more than a third reported complete pain resolution
- 73% reduction in active opioid use
- High patient satisfaction with the procedure
- 79% indicated they would have the procedure again for the same condition
- 65% had resumed the level of activity they enjoyed prior to low back pain
“Following publication of two Level I Randomized Control Trials, the SMART study and the INTRACEPT study, we are pleased to see such a meaningful degree of clinical improvement being maintained more than five years after patients received the Intracept procedure,” said Art Taylor, President & CEO of Relievant Medsystems. “This unparalleled level of clinical evidence demonstrating that Intracept is a highly-effective and durable therapy with a very favorable safety profile, is strongly compelling for physicians, payers, and most of all, patients suffering from vertebrogenic CLBP.”

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







