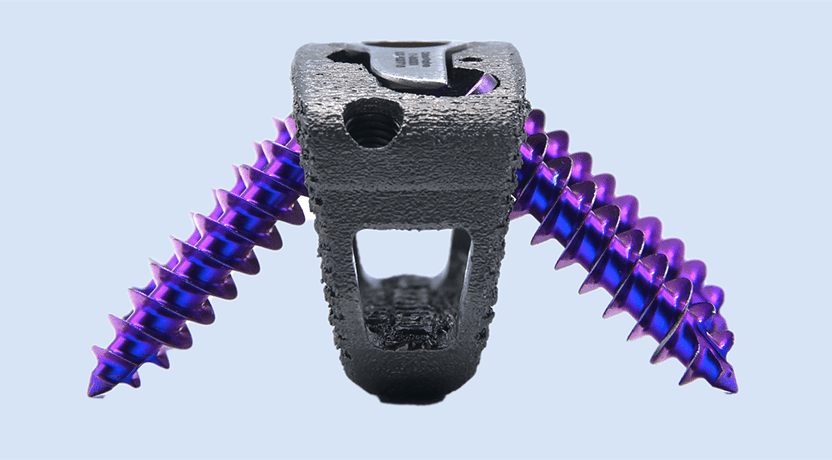






Acuity Surgical Devices received a second special FDA 510(k) clearance of a medical device featuring Promimic’s HAnano Surface.
The Align portfolio includes additively manufactured titanium devices intended for the following approaches: stand-alone Anterior Lumbar Interbody Fusion, Oblique Lumbar Interbody Fusion, and Lateral Lumbar Interbody Fusion. Each Align device features an enhanced porous structure designed to improve adhesion to the vertebral body endplates. With this special 510(k) clearance, Align implants also integrate the super-hydrophilic HAnano Surface that has been shown to promote faster and stronger osseointegration in pre-clinical studies with roughened titanium implants.
HAnano Surface has been evaluated in more than 30 preclinical studies, testing a variety of implant materials and designs in different models. It has proven to increase anchoring strength for titanium implants by up to 35% in just three weeks. The studies also show that HAnano Surface up-regulates important bone marker proteins and improves new bone formation on titanium implant surface by 67% at four weeks.
“We are delighted that Acuity Surgical Devices chose our hydrophilic HAnano Surface to further differentiate their product portfolio. That the clearance came after an expedited special 510(k) further establishes a faster path to market for our customers, so that more implants with improved osseointegration can be used clinically,” said Magnus Larsson, Promimic CEO.
“We believe the bioactive performance of the nano surface supplementing our enhanced porous structure will make Align implants with HAnano Surface the new gold standard in stability,” said John Davidson, President of Acuity Surgical Devices.
Source: Promimic
Acuity Surgical Devices received a second special FDA 510(k) clearance of a medical device featuring Promimic's HAnano Surface.
The Align portfolio includes additively manufactured titanium devices intended for the following approaches: stand-alone Anterior Lumbar Interbody Fusion, Oblique Lumbar Interbody Fusion, and Lateral Lumbar Interbody...
Acuity Surgical Devices received a second special FDA 510(k) clearance of a medical device featuring Promimic’s HAnano Surface.
The Align portfolio includes additively manufactured titanium devices intended for the following approaches: stand-alone Anterior Lumbar Interbody Fusion, Oblique Lumbar Interbody Fusion, and Lateral Lumbar Interbody Fusion. Each Align device features an enhanced porous structure designed to improve adhesion to the vertebral body endplates. With this special 510(k) clearance, Align implants also integrate the super-hydrophilic HAnano Surface that has been shown to promote faster and stronger osseointegration in pre-clinical studies with roughened titanium implants.
HAnano Surface has been evaluated in more than 30 preclinical studies, testing a variety of implant materials and designs in different models. It has proven to increase anchoring strength for titanium implants by up to 35% in just three weeks. The studies also show that HAnano Surface up-regulates important bone marker proteins and improves new bone formation on titanium implant surface by 67% at four weeks.
“We are delighted that Acuity Surgical Devices chose our hydrophilic HAnano Surface to further differentiate their product portfolio. That the clearance came after an expedited special 510(k) further establishes a faster path to market for our customers, so that more implants with improved osseointegration can be used clinically,” said Magnus Larsson, Promimic CEO.
“We believe the bioactive performance of the nano surface supplementing our enhanced porous structure will make Align implants with HAnano Surface the new gold standard in stability,” said John Davidson, President of Acuity Surgical Devices.
Source: Promimic


You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







