Copy to clipboard
Copy to clipboard 
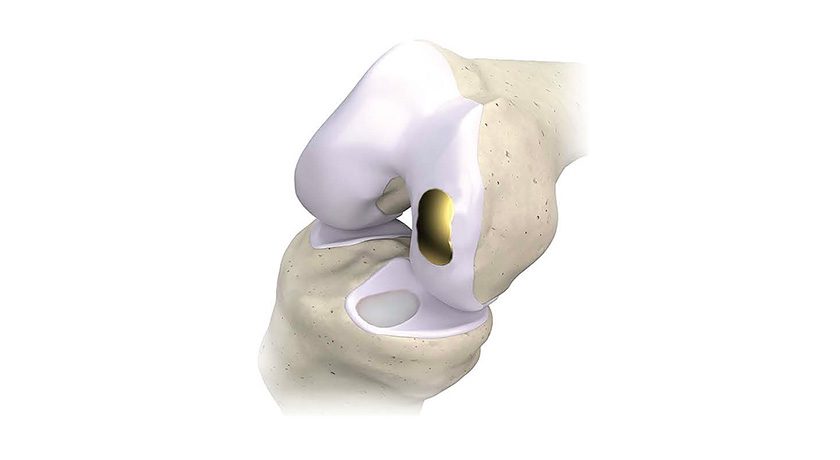
Regentis Biomaterials received CE Mark approval for its GelrinC® biodegradable implant, made with denatured human fibrinogen, for the treatment of articular knee cartilage defects. This expands upon the existing CE Mark that is based upon denatured bovine-sourced fibrinogen.
Regentis is conducting an FDA-approved Phase III pivotal multi-site clinical trial on GelrinC in the U.S. and Europe to support a premarket approval application.
Sources: Regentis Biomaterials Ltd.; ORTHOWORLD Inc.
Regentis Biomaterials received CE Mark approval for its GelrinC® biodegradable implant, made with denatured human fibrinogen, for the treatment of articular knee cartilage defects. This expands upon the existing CE Mark that is based upon denatured bovine-sourced fibrinogen.
Regentis is conducting an FDA-approved Phase III pivotal...
Regentis Biomaterials received CE Mark approval for its GelrinC® biodegradable implant, made with denatured human fibrinogen, for the treatment of articular knee cartilage defects. This expands upon the existing CE Mark that is based upon denatured bovine-sourced fibrinogen.
Regentis is conducting an FDA-approved Phase III pivotal multi-site clinical trial on GelrinC in the U.S. and Europe to support a premarket approval application.
Sources: Regentis Biomaterials Ltd.; ORTHOWORLD Inc.

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.