Copy to clipboard
Copy to clipboard 
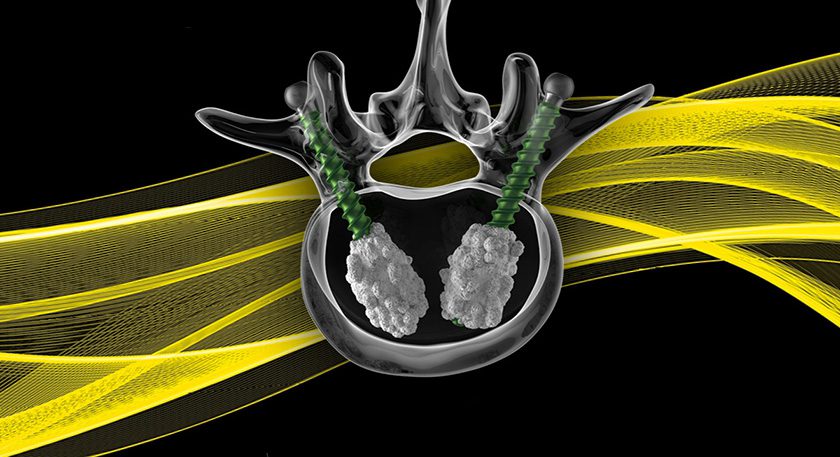
Regentis Biomaterials expanded the SAGE clinical trial of GelrinC™ to 11 U.S. sites, comparing the device to microfracture in the treatment of damaged articular knee cartilage.
SAGE is an FDA Investigational Device Exemption multi-center Phase III pivotal study that will enroll 120 patients. GelrinC contains synthetic polyethylene glycol material and a structurally modified form of human fibrinogen protein. The device is implanted as a liquid to completely fill a defect, and is cured into a gel upon which the body’s own stem cells settle. Over time, GelrinC is resorbed and replaced by new cartilage tissue.
Source: Regentis Biomaterials
Regentis Biomaterials expanded the SAGE clinical trial of GelrinC™ to 11 U.S. sites, comparing the device to microfracture in the treatment of damaged articular knee cartilage.
SAGE is an FDA Investigational Device Exemption multi-center Phase III pivotal study that will enroll 120 patients. GelrinC contains synthetic polyethylene glycol...
Regentis Biomaterials expanded the SAGE clinical trial of GelrinC™ to 11 U.S. sites, comparing the device to microfracture in the treatment of damaged articular knee cartilage.
SAGE is an FDA Investigational Device Exemption multi-center Phase III pivotal study that will enroll 120 patients. GelrinC contains synthetic polyethylene glycol material and a structurally modified form of human fibrinogen protein. The device is implanted as a liquid to completely fill a defect, and is cured into a gel upon which the body’s own stem cells settle. Over time, GelrinC is resorbed and replaced by new cartilage tissue.
Source: Regentis Biomaterials

You’ve reached your limit.
We’re glad you’re finding value in our content — and we’d love for you to keep going.
Subscribe now for unlimited access to orthopedic business intelligence.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.