
 Copy to clipboard
Copy to clipboard 
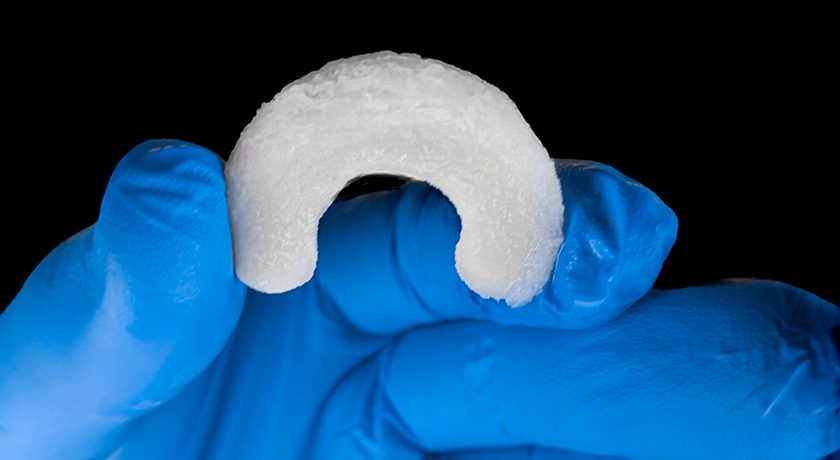
Regenity Biosciences received FDA 510(k) clearance to market RejuvaKnee, a minimally invasive collagen-based meniscal implant indicated for use in the reinforcement and repair of soft tissue injuries.
RejuvaKnee allows for a more complete recovery by facilitating the regeneration of the native meniscal tissue instead of cutting or replacing it. In a 12-month animal study that examined biochemical and biomechanical attributes and histology for tissue growth, RejuvaKnee demonstrated that in three months, the regenerated meniscus is able to withstand full weight bearing, and the knee returns to normal range of motion. These results are superior to that of meniscectomy and allograft transplantation. Neovascularization and tissue histology also demonstrated healthy tissue regeneration with RejuvaKnee achieving nearly five times more tissue growth than the standard of care. Meniscectomy can offer high short-term clinical success rates but can frequently lead to follow-on meniscectomy or predisposition for osteoarthritis, as opposed to RejuvaKnee, which is designed to elongate the life of a knee that has been impacted by meniscal injury.
RejuvaKnee is a natural bovine-derived collagen implant that facilitates host tissue ingrowth and repair of damaged or injured meniscal tissue. In human cadaver studies, Regenity, in partnership with a panel of key opinion leaders in sports medicine, demonstrated the application and ease of use for arthroscopic implantation using a wide range of instrumentation currently available on the market.
“The FDA clearance of RejuvaKnee represents a turning point in the meniscal repair market and marks an exciting innovation milestone for Regenity as a global leader in regenerative medicine solutions,” said Shawn McCarthy, CEO of Regenity Biosciences. “The pre-clinical animal study data supports our confidence that RejuvaKnee will deliver results by facilitating significant tissue regeneration and balanced implant resorption. As the only FDA-cleared regenerative implant device for meniscal repair on the market, this is a game changer for the industry given it will be indicated for a significant portion of the more than one million meniscectomies that are performed each year in the United States. With this innovation, our 60th 510(k) approval, we are further expanding the Regenity product portfolio that we bring to our commercial partners to improve patient outcomes.”
Regenity is currently exploring opportunities via a strategic partner to commercialize the device in the United States.
Source: Regenity Biosciences
Regenity Biosciences received FDA 510(k) clearance to market RejuvaKnee, a minimally invasive collagen-based meniscal implant indicated for use in the reinforcement and repair of soft tissue injuries.
RejuvaKnee allows for a more complete recovery by facilitating the regeneration of the native meniscal tissue instead of cutting or replacing...
Regenity Biosciences received FDA 510(k) clearance to market RejuvaKnee, a minimally invasive collagen-based meniscal implant indicated for use in the reinforcement and repair of soft tissue injuries.
RejuvaKnee allows for a more complete recovery by facilitating the regeneration of the native meniscal tissue instead of cutting or replacing it. In a 12-month animal study that examined biochemical and biomechanical attributes and histology for tissue growth, RejuvaKnee demonstrated that in three months, the regenerated meniscus is able to withstand full weight bearing, and the knee returns to normal range of motion. These results are superior to that of meniscectomy and allograft transplantation. Neovascularization and tissue histology also demonstrated healthy tissue regeneration with RejuvaKnee achieving nearly five times more tissue growth than the standard of care. Meniscectomy can offer high short-term clinical success rates but can frequently lead to follow-on meniscectomy or predisposition for osteoarthritis, as opposed to RejuvaKnee, which is designed to elongate the life of a knee that has been impacted by meniscal injury.
RejuvaKnee is a natural bovine-derived collagen implant that facilitates host tissue ingrowth and repair of damaged or injured meniscal tissue. In human cadaver studies, Regenity, in partnership with a panel of key opinion leaders in sports medicine, demonstrated the application and ease of use for arthroscopic implantation using a wide range of instrumentation currently available on the market.
“The FDA clearance of RejuvaKnee represents a turning point in the meniscal repair market and marks an exciting innovation milestone for Regenity as a global leader in regenerative medicine solutions,” said Shawn McCarthy, CEO of Regenity Biosciences. “The pre-clinical animal study data supports our confidence that RejuvaKnee will deliver results by facilitating significant tissue regeneration and balanced implant resorption. As the only FDA-cleared regenerative implant device for meniscal repair on the market, this is a game changer for the industry given it will be indicated for a significant portion of the more than one million meniscectomies that are performed each year in the United States. With this innovation, our 60th 510(k) approval, we are further expanding the Regenity product portfolio that we bring to our commercial partners to improve patient outcomes.”
Regenity is currently exploring opportunities via a strategic partner to commercialize the device in the United States.
Source: Regenity Biosciences

You’ve reached your limit.
We’re glad you’re finding value in our content — and we’d love for you to keep going.
Subscribe now for unlimited access to orthopedic business intelligence.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.