
 Copy to clipboard
Copy to clipboard 
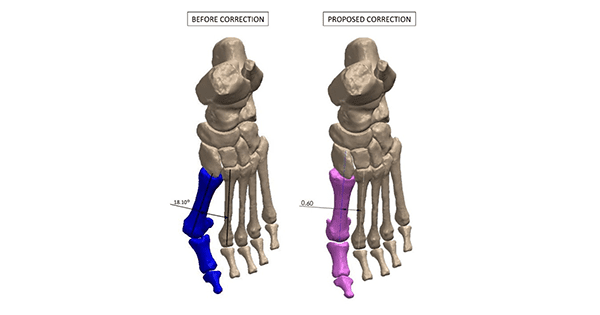
Red Point Medical 3D (RPM-3D) has been granted FDA 510(k) clearance to market patient-specific bone cutting guides for use in foot and ankle reconstructive surgeries, such as bunion correction. This reportedly represents the first patient-specific instrumentation (PSI)-based company to gain FDA clearance for patient-specific bone cutting guides for use in foot and ankle reconstructive surgeries.
RPM-3D’s patent-pending patient-specific surgical guide system utilizes proprietary artificial intelligence software (RedPoint Intelligence™) to convert data derived from a CT scan of the patient’s foot and/or ankle into a 3D model, which is then manipulated to resemble the desired correction as prescribed by the surgeon.
Next, RPM-3D provides the surgeon with the opportunity to rehearse the surgery on a 3D-printed model, provides them with a fully customized technique guide, and a 3D-printed custom surgical cut guide that is optimized and personalized to correct complex surgical deformity with the ultimate precision.
The first family of RPM-3D customized guides will be marketed as The Better Bunion™, which is ready for U.S. launch. This will target simplification of surgical steps associated with bunion corrective techniques (Lapidus, MIS, Akins). Better Bunion is designed for precise bone osteotomies and deformity reduction, and decreased operating room, tourniquet, anesthesia and radiographic exposure times relative to other generic instrumented bunion corrective procedures available on the market.
Better Bunion Lapidus allows surgeons to utilize only six steps to prepare the bone before focusing on their preferred fixation. Most current instrumented systems on the market require 15 to 22 steps and the use of proprietary hardware.
Source: RedPoint Medical 3D
Red Point Medical 3D (RPM-3D) has been granted FDA 510(k) clearance to market patient-specific bone cutting guides for use in foot and ankle reconstructive surgeries, such as bunion correction. This reportedly represents the first patient-specific instrumentation (PSI)-based company to gain FDA clearance for patient-specific bone cutting guides...
Red Point Medical 3D (RPM-3D) has been granted FDA 510(k) clearance to market patient-specific bone cutting guides for use in foot and ankle reconstructive surgeries, such as bunion correction. This reportedly represents the first patient-specific instrumentation (PSI)-based company to gain FDA clearance for patient-specific bone cutting guides for use in foot and ankle reconstructive surgeries.
RPM-3D’s patent-pending patient-specific surgical guide system utilizes proprietary artificial intelligence software (RedPoint Intelligence™) to convert data derived from a CT scan of the patient’s foot and/or ankle into a 3D model, which is then manipulated to resemble the desired correction as prescribed by the surgeon.
Next, RPM-3D provides the surgeon with the opportunity to rehearse the surgery on a 3D-printed model, provides them with a fully customized technique guide, and a 3D-printed custom surgical cut guide that is optimized and personalized to correct complex surgical deformity with the ultimate precision.
The first family of RPM-3D customized guides will be marketed as The Better Bunion™, which is ready for U.S. launch. This will target simplification of surgical steps associated with bunion corrective techniques (Lapidus, MIS, Akins). Better Bunion is designed for precise bone osteotomies and deformity reduction, and decreased operating room, tourniquet, anesthesia and radiographic exposure times relative to other generic instrumented bunion corrective procedures available on the market.
Better Bunion Lapidus allows surgeons to utilize only six steps to prepare the bone before focusing on their preferred fixation. Most current instrumented systems on the market require 15 to 22 steps and the use of proprietary hardware.
Source: RedPoint Medical 3D

You’ve reached your limit.
We’re glad you’re finding value in our content — and we’d love for you to keep going.
Subscribe now for unlimited access to orthopedic business intelligence.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







