
 Copy to clipboard
Copy to clipboard 
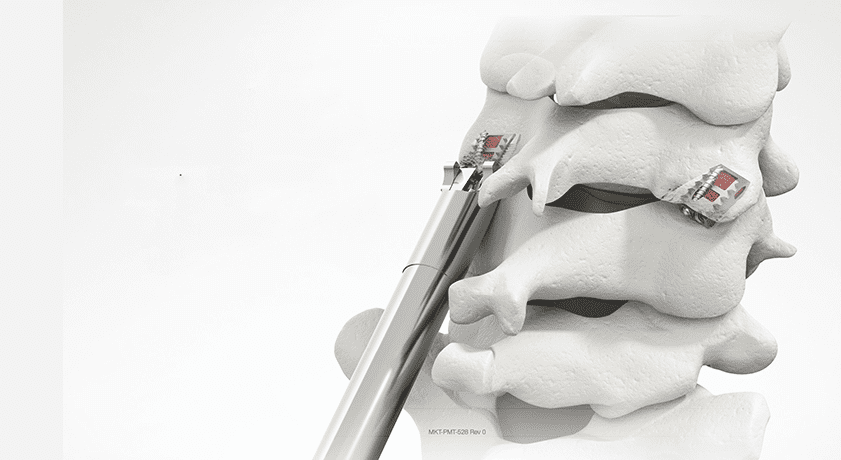
Providence Medical Technology received FDA 510(k) clearance to market the CAVUX® Facet Fixation System (FFS) for a new indication. The system is a novel integrated cage and screw system that is implanted bilaterally in the facet joints to provide stabilization until fusion occurs.
CAVUX FFS is now indicated for patients requiring a revision for an anterior pseudarthrosis at one level, from C3 to C7, with autogenous and/or allogenic bone graft. CAVUX FFS is now specifically cleared as an adjunct to posterior cervical fusion for anterior cervical fusion revision surgery, a follow-up procedure after an initial surgery failed to resolve symptoms or properly heal.
The FDA clearance was based on results from the Providence-sponsored REVISE Clinical Study, which summarized Real World Evidence pooled across six clinical sites treating patients with CAVUX FFS to revise a prior Anterior Cervical Discectomy & Fusion (ACDF). The dataset consisted of 191 cases reviewed, with half of the patients returning for radiographic and clinical assessments.
Long-term outcomes were collected over an average of 39 months from revision. In the cohort of single-level pseudarthrosis patients returning for follow-up, 96% were determined by the surgeon to be fused and none required a subsequent surgical intervention. Additionally, an independent core lab reported that 92% had a segmental range of motion less than 2 degrees and 75% had continuous bridging bone verified by CT scan. CAVUX FFS is implanted using the company’s flagship CORUS™ Spinal System, which enables a surgeon to perform a tissue-sparing posterior cervical fusion before placing implants.
Providence Medical Technology received FDA 510(k) clearance to market the CAVUX® Facet Fixation System (FFS) for a new indication. The system is a novel integrated cage and screw system that is implanted bilaterally in the facet joints to provide stabilization until fusion occurs.
CAVUX FFS is now indicated for patients requiring a revision...
Providence Medical Technology received FDA 510(k) clearance to market the CAVUX® Facet Fixation System (FFS) for a new indication. The system is a novel integrated cage and screw system that is implanted bilaterally in the facet joints to provide stabilization until fusion occurs.
CAVUX FFS is now indicated for patients requiring a revision for an anterior pseudarthrosis at one level, from C3 to C7, with autogenous and/or allogenic bone graft. CAVUX FFS is now specifically cleared as an adjunct to posterior cervical fusion for anterior cervical fusion revision surgery, a follow-up procedure after an initial surgery failed to resolve symptoms or properly heal.
The FDA clearance was based on results from the Providence-sponsored REVISE Clinical Study, which summarized Real World Evidence pooled across six clinical sites treating patients with CAVUX FFS to revise a prior Anterior Cervical Discectomy & Fusion (ACDF). The dataset consisted of 191 cases reviewed, with half of the patients returning for radiographic and clinical assessments.
Long-term outcomes were collected over an average of 39 months from revision. In the cohort of single-level pseudarthrosis patients returning for follow-up, 96% were determined by the surgeon to be fused and none required a subsequent surgical intervention. Additionally, an independent core lab reported that 92% had a segmental range of motion less than 2 degrees and 75% had continuous bridging bone verified by CT scan. CAVUX FFS is implanted using the company’s flagship CORUS™ Spinal System, which enables a surgeon to perform a tissue-sparing posterior cervical fusion before placing implants.
Source: Providence Medical Technology, Inc.

You’ve reached your limit.
We’re glad you’re finding value in our content — and we’d love for you to keep going.
Subscribe now for unlimited access to orthopedic business intelligence.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







