
 Copy to clipboard
Copy to clipboard 
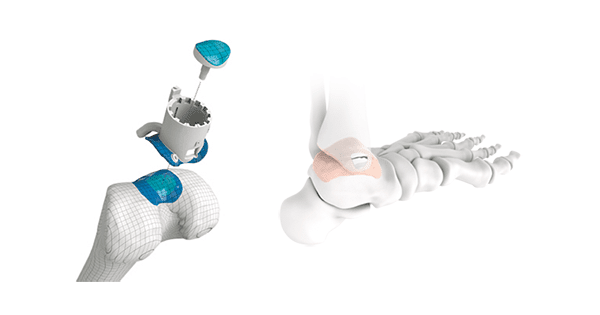
Episurf Medical today announces that the first surgery with the ankle implant Episealer® Talus in Spain has been scheduled.
“We are continuing to execute on our international strategy, and I am pleased with having the first Episealer Talus surgery in Spain scheduled. This also means that during 2021, we have had customers in 15 countries globally. Given that we are still early on our development curve, I believe that says something about our level om ambition,” saud Pål Ryfors, CEO.
Further, results from a multicentre study with 5-7 years’ follow-up of Episealer® patients have been accepted for publication in the scientific journal Archives of Orthopaedic and Trauma Surgery. The publication with the title “Good subjective outcome and low risk of revision surgery with a novel customized metal implant for focal femoral chondral lesions at a follow-up after a minimum of five years” is based on the results from a prospective, consecutive cohort study with the objective to assess subjective and objective outcome at a minimum of five years after implantation of an Episealer® Knee implant. The publication is expected to be available online shortly.
The Episealer® Knee implant is intended for treatment of focal cartilage and underlying bone defects on the femoral side of the knee joint. These defects often imply severe pain, which significantly can impact the patient’s quality of life. Current standard of care, which includes various biological treatment alternatives, has shown limited effectiveness, especially with increasing age.
The study was initiated in 2012 and has focused on the clinical outcome for the 10 very first Swedish Episealer Knee patients.
Source: Episurf Medical
Episurf Medical today announces that the first surgery with the ankle implant Episealer® Talus in Spain has been scheduled.
“We are continuing to execute on our international strategy, and I am pleased with having the first Episealer Talus surgery in Spain scheduled. This also means that during 2021, we have had customers in 15 countries...
Episurf Medical today announces that the first surgery with the ankle implant Episealer® Talus in Spain has been scheduled.
“We are continuing to execute on our international strategy, and I am pleased with having the first Episealer Talus surgery in Spain scheduled. This also means that during 2021, we have had customers in 15 countries globally. Given that we are still early on our development curve, I believe that says something about our level om ambition,” saud Pål Ryfors, CEO.
Further, results from a multicentre study with 5-7 years’ follow-up of Episealer® patients have been accepted for publication in the scientific journal Archives of Orthopaedic and Trauma Surgery. The publication with the title “Good subjective outcome and low risk of revision surgery with a novel customized metal implant for focal femoral chondral lesions at a follow-up after a minimum of five years” is based on the results from a prospective, consecutive cohort study with the objective to assess subjective and objective outcome at a minimum of five years after implantation of an Episealer® Knee implant. The publication is expected to be available online shortly.
The Episealer® Knee implant is intended for treatment of focal cartilage and underlying bone defects on the femoral side of the knee joint. These defects often imply severe pain, which significantly can impact the patient’s quality of life. Current standard of care, which includes various biological treatment alternatives, has shown limited effectiveness, especially with increasing age.
The study was initiated in 2012 and has focused on the clinical outcome for the 10 very first Swedish Episealer Knee patients.
Source: Episurf Medical

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







