
 Copy to clipboard
Copy to clipboard 
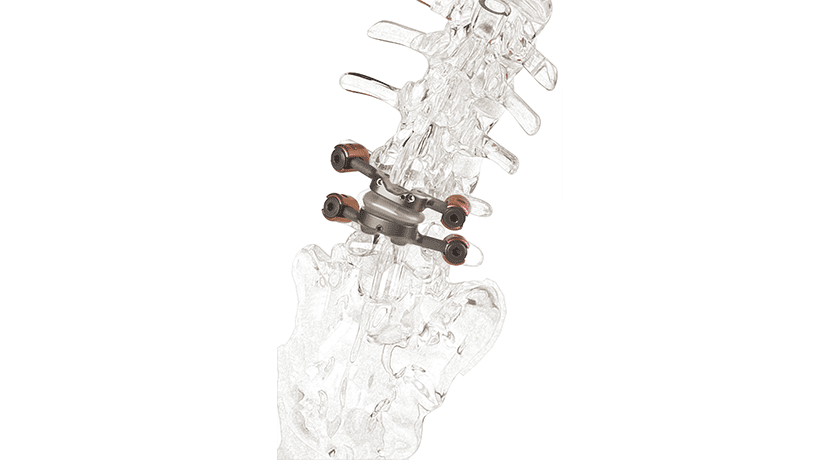
One-year outcomes from a clinical trial of Premia Spine’s TOPS facet replacement system found that lumbar facet arthroplasty with the TOPS device demonstrated a statistically significant improvement in all patient-reported outcome measures, a low surgical complication rate and the ability to maintain motion at the index level while limiting sagittal translation.
The objective of the prospective, randomized, multicenter U.S. FDA investigational device exemption (IDE) trial is to compare the two-year clinical and radiographic outcomes and safety profile of patients undergoing either a lumbar TOPS facet arthroplasty or a transforaminal lumbar interbody fusion (TLIF). This is the first analysis of early clinical outcomes and complications from the TOPS IDE trial.
In the study, 153 patients were enrolled in the investigational arm of the clinical trial and underwent implantation of the TOPS device, with 145 TOPS devices implanted at L4-5, and eight devices implanted at L3-4. Of those, 69% reached their one-year follow-up at the time of this interim assessment, and were included in the PROMs and radiographic analyses.
Lumbar facet arthroplasty with the TOPS device demonstrated a low complication rate, significant improvement in all PROMs, and the ability to maintain motion at the index level while limiting sagittal translation. The complication rates and improved postoperative PROMs are similar to those reported in the literature following single-level TLIF. No patient in the TOPS cohort required reoperation within the first year owing to adjacent segment degeneration.
As an alternative to standard fusion, TOPS is designed to maintain motion at a previously pathologic intervertebral segment.
Source: Premia Spine
One-year outcomes from a clinical trial of Premia Spine's TOPS facet replacement system found that lumbar facet arthroplasty with the TOPS device demonstrated a statistically significant improvement in all patient-reported outcome measures, a low surgical complication rate and the ability to maintain motion at the index level while limiting...
One-year outcomes from a clinical trial of Premia Spine’s TOPS facet replacement system found that lumbar facet arthroplasty with the TOPS device demonstrated a statistically significant improvement in all patient-reported outcome measures, a low surgical complication rate and the ability to maintain motion at the index level while limiting sagittal translation.
The objective of the prospective, randomized, multicenter U.S. FDA investigational device exemption (IDE) trial is to compare the two-year clinical and radiographic outcomes and safety profile of patients undergoing either a lumbar TOPS facet arthroplasty or a transforaminal lumbar interbody fusion (TLIF). This is the first analysis of early clinical outcomes and complications from the TOPS IDE trial.
In the study, 153 patients were enrolled in the investigational arm of the clinical trial and underwent implantation of the TOPS device, with 145 TOPS devices implanted at L4-5, and eight devices implanted at L3-4. Of those, 69% reached their one-year follow-up at the time of this interim assessment, and were included in the PROMs and radiographic analyses.
Lumbar facet arthroplasty with the TOPS device demonstrated a low complication rate, significant improvement in all PROMs, and the ability to maintain motion at the index level while limiting sagittal translation. The complication rates and improved postoperative PROMs are similar to those reported in the literature following single-level TLIF. No patient in the TOPS cohort required reoperation within the first year owing to adjacent segment degeneration.
As an alternative to standard fusion, TOPS is designed to maintain motion at a previously pathologic intervertebral segment.
Source: Premia Spine

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







