
 Copy to clipboard
Copy to clipboard 
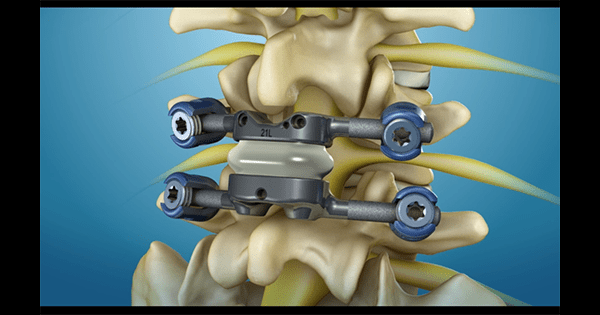
Premia Spine secured FDA approval for a pivotal Investigational Device Exemption (IDE) study of the new TOPS™ system, a posterior arthroplasty device for the treatment of degenerative grade I spondylolisthesis and stenosis. The study seeks to establish the superiority of TOPS vs. traditional lumbar spinal fusion.
The IDE study will take place in 30 institutions and enroll 330 subjects. Patients will be randomized to either TOPS or lumbar fusion (i.e., an interbody cage plus screws and rods), with a 67% likelihood of receiving the TOPS device. Additional spine research centers are preparing institutional review board submissions to join the study.
The new TOPS device, with a 30% smaller footprint and a simpler surgical technique from the original, has been in commercial use in Europe for over five years.
Premia Spine licensed TOPS technology from Impliant in 2011. Over $100MM has been invested to design, develop and commercialize TOPS, with >12 years of clinical use in 1,000 patients.
Premia also markets the FDA-cleared ProMIS™ Fixation System, which features a choice of screw placement techniques in one instrument kit.
Sources: Premia Spine, Ltd.; ORTHOWORLD Inc.
Premia Spine secured FDA approval for a pivotal Investigational Device Exemption (IDE) study of the new TOPS™ system, a posterior arthroplasty device for the treatment of degenerative grade I spondylolisthesis and stenosis. The study seeks to establish the superiority of TOPS vs. traditional lumbar spinal fusion.
The IDE study will take place...
Premia Spine secured FDA approval for a pivotal Investigational Device Exemption (IDE) study of the new TOPS™ system, a posterior arthroplasty device for the treatment of degenerative grade I spondylolisthesis and stenosis. The study seeks to establish the superiority of TOPS vs. traditional lumbar spinal fusion.
The IDE study will take place in 30 institutions and enroll 330 subjects. Patients will be randomized to either TOPS or lumbar fusion (i.e., an interbody cage plus screws and rods), with a 67% likelihood of receiving the TOPS device. Additional spine research centers are preparing institutional review board submissions to join the study.
The new TOPS device, with a 30% smaller footprint and a simpler surgical technique from the original, has been in commercial use in Europe for over five years.
Premia Spine licensed TOPS technology from Impliant in 2011. Over $100MM has been invested to design, develop and commercialize TOPS, with >12 years of clinical use in 1,000 patients.
Premia also markets the FDA-cleared ProMIS™ Fixation System, which features a choice of screw placement techniques in one instrument kit.
Sources: Premia Spine, Ltd.; ORTHOWORLD Inc.

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.