
 Copy to clipboard
Copy to clipboard 
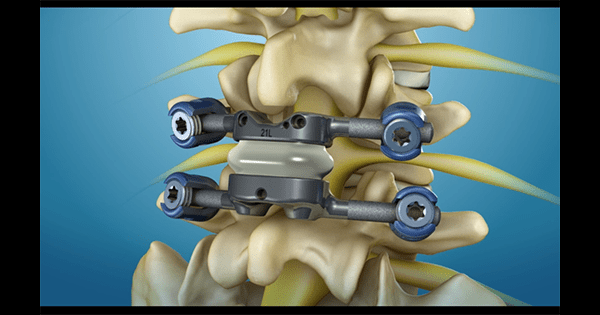
Premia Spine launched its FDA-approved U.S. pivotal Investigational Device Exemption study of the TOPS™ posterior arthroplasty device vs. traditional lumbar fusion in the treatment of degenerative grade I spondylolisthesis and spinal stenosis.
The randomized study will enroll 330 patients at 30 clinical sites, measuring ODI, VAS, neurologic function, device integrity, reoperation rates and other quantitative outcomes. The study seeks to establish superiority of TOPS vs. fusion.
Sources: Premia Spine; ORTHOWORLD Inc.
Premia Spine launched its FDA-approved U.S. pivotal Investigational Device Exemption study of the TOPS™ posterior arthroplasty device vs. traditional lumbar fusion in the treatment of degenerative grade I spondylolisthesis and spinal stenosis.
The randomized study will enroll 330 patients at 30 clinical sites, measuring ODI, VAS,...
Premia Spine launched its FDA-approved U.S. pivotal Investigational Device Exemption study of the TOPS™ posterior arthroplasty device vs. traditional lumbar fusion in the treatment of degenerative grade I spondylolisthesis and spinal stenosis.
The randomized study will enroll 330 patients at 30 clinical sites, measuring ODI, VAS, neurologic function, device integrity, reoperation rates and other quantitative outcomes. The study seeks to establish superiority of TOPS vs. fusion.
Sources: Premia Spine; ORTHOWORLD Inc.

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







