
 Copy to clipboard
Copy to clipboard 
Precision Spine was granted FDA 510(k) clearance to market the Dakota ACDF™ Standalone System to treat degenerative disc disease.
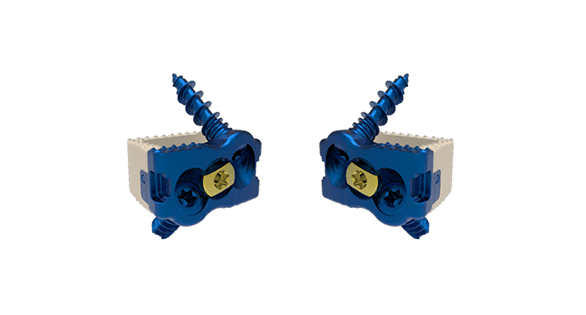
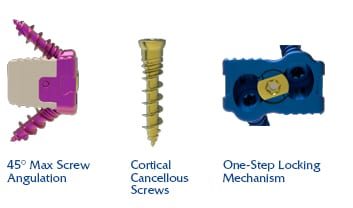 Dakota ACDF comprises a titanium plate polyetheretherketone cage with cortical cancellous screws and a large cavity for autogenous bone graft to support fusion.
Dakota ACDF comprises a titanium plate polyetheretherketone cage with cortical cancellous screws and a large cavity for autogenous bone graft to support fusion.
The device is placed via an anterior approach at the C2 to T1 disc levels. Patients should have at least six weeks of non-operative treatment prior to treatment with an intervertebral fusion device.
“The Dakota ACDF System represents another example of how Precision Spine is making good on its commitment to develop surgeon-designed devices that embody the advanced features surgeons need to help improve OR efficiency and achieve positive patient outcomes,” said Chris DeNicola, Chief Operating Officer of Precision Spine.
Precision Spine was granted FDA 510(k) clearance to market the Dakota ACDF™ Standalone System to treat degenerative disc disease.
Dakota ACDF comprises a titanium plate polyetheretherketone cage with cortical cancellous screws and a large cavity for autogenous bone graft to support fusion.
The device is placed via an anterior...
Precision Spine was granted FDA 510(k) clearance to market the Dakota ACDF™ Standalone System to treat degenerative disc disease.
 Dakota ACDF comprises a titanium plate polyetheretherketone cage with cortical cancellous screws and a large cavity for autogenous bone graft to support fusion.
Dakota ACDF comprises a titanium plate polyetheretherketone cage with cortical cancellous screws and a large cavity for autogenous bone graft to support fusion.
The device is placed via an anterior approach at the C2 to T1 disc levels. Patients should have at least six weeks of non-operative treatment prior to treatment with an intervertebral fusion device.
“The Dakota ACDF System represents another example of how Precision Spine is making good on its commitment to develop surgeon-designed devices that embody the advanced features surgeons need to help improve OR efficiency and achieve positive patient outcomes,” said Chris DeNicola, Chief Operating Officer of Precision Spine.

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







