
 Copy to clipboard
Copy to clipboard 
Study results indicate that the use of Intrinsic Therapeutics’ Barricaid in high-risk patients results in significantly fewer reherniations and reoperations when compared to patients treated with discectomy alone.
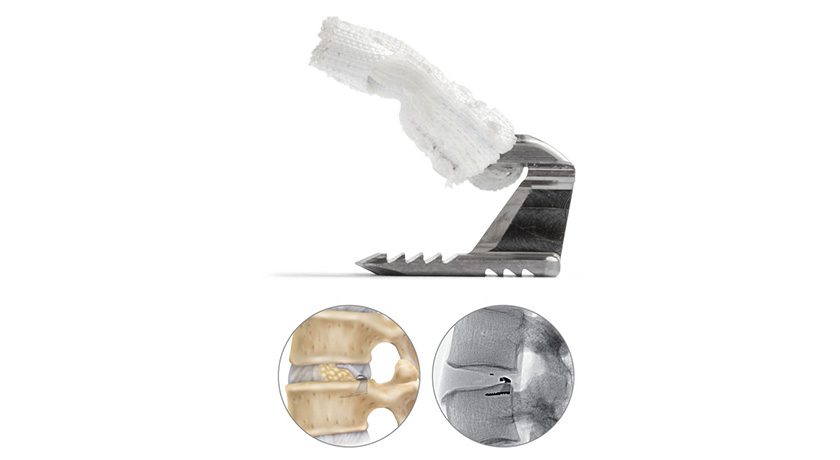
Barricaid is a proprietary technology designed to prevent reherniation and reoperation in patients with large annular defects following lumbar discectomy.
The study compared the results of a 55-patient, single-arm, postmarket study to the 554 patient, level-1, randomized, superiority PMA trial. The results of this study demonstrated a statistically significant reduction of reherniation or reoperation when utilizing bone-anchored annular closure.
This study also provided evidence that the Barricaid procedure can lower opioid use and costly hospital readmissions by reducing reherniations. Another key metric was that nearly all the Barricaid patients returned to work in under three weeks.
Barricaid received FDA premarket approval in 2019. In 2020, Centers for Medicare and Medicaid Services issued a C-code for hospitals and surgery centers to report for billing and payment, and in late 2022, the Centers for Disease Control issued ICD-10 codes to track and monitor defect size in discectomy patients.
Cary Hagan, CEO of Intrinsic, said, “Publication of this study further validates the already robust body of clinical evidence for Barricaid and is the eighth distinct patient population studied and recorded in the scientific literature. There is sufficient evidence to support medical necessity and for payors to remove the label of ‘investigational/experimental’ and differentiate Barricaid from prior annular repair technologies,” as recently recognized by Cigna in their positive coverage policy. “I expect other private insurers will begin to update their coverage policies for Barricaid based on this confirmatory evidence.”
Source: Intrinsic Therapeutics, Inc.
Study results indicate that the use of Intrinsic Therapeutics' Barricaid in high-risk patients results in significantly fewer reherniations and reoperations when compared to patients treated with discectomy alone.
Barricaid is a proprietary technology designed to prevent reherniation and reoperation in patients with large annular defects...
Study results indicate that the use of Intrinsic Therapeutics’ Barricaid in high-risk patients results in significantly fewer reherniations and reoperations when compared to patients treated with discectomy alone.
Barricaid is a proprietary technology designed to prevent reherniation and reoperation in patients with large annular defects following lumbar discectomy.
The study compared the results of a 55-patient, single-arm, postmarket study to the 554 patient, level-1, randomized, superiority PMA trial. The results of this study demonstrated a statistically significant reduction of reherniation or reoperation when utilizing bone-anchored annular closure.
This study also provided evidence that the Barricaid procedure can lower opioid use and costly hospital readmissions by reducing reherniations. Another key metric was that nearly all the Barricaid patients returned to work in under three weeks.
Barricaid received FDA premarket approval in 2019. In 2020, Centers for Medicare and Medicaid Services issued a C-code for hospitals and surgery centers to report for billing and payment, and in late 2022, the Centers for Disease Control issued ICD-10 codes to track and monitor defect size in discectomy patients.
Cary Hagan, CEO of Intrinsic, said, “Publication of this study further validates the already robust body of clinical evidence for Barricaid and is the eighth distinct patient population studied and recorded in the scientific literature. There is sufficient evidence to support medical necessity and for payors to remove the label of ‘investigational/experimental’ and differentiate Barricaid from prior annular repair technologies,” as recently recognized by Cigna in their positive coverage policy. “I expect other private insurers will begin to update their coverage policies for Barricaid based on this confirmatory evidence.”
Source: Intrinsic Therapeutics, Inc.

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







