Copy to clipboard
Copy to clipboard 
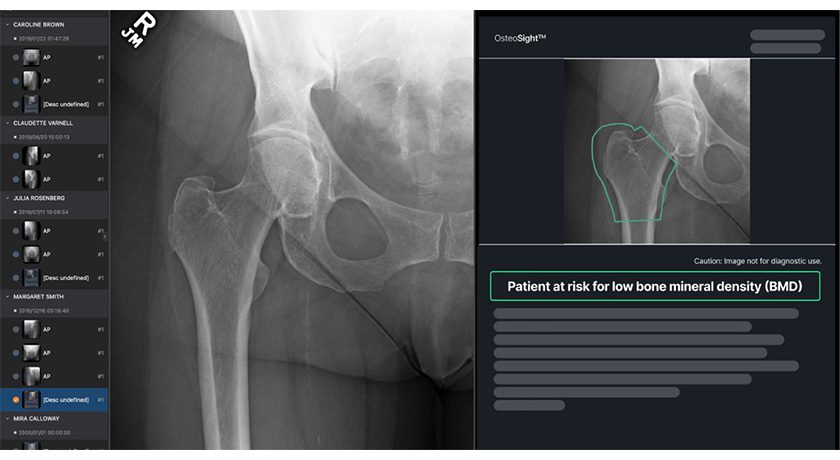
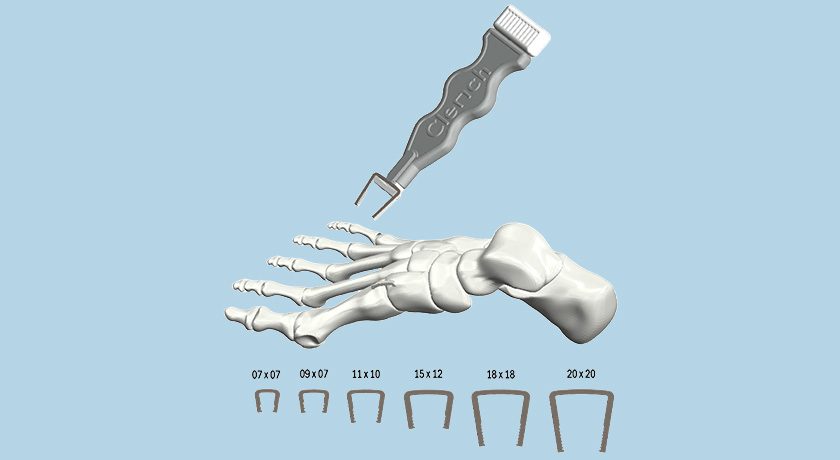
Aurora Spine’s proprietary implant locking technology, covered by U.S. Patent No. 9,603,637 (Polyaxial Interspinous Fusion Implant and Bone Growth Stimulation System), is available for out-licensing or private labeling opportunities.
Leadership notes that the device, with its combination of a ONE-STEP™ locking mechanism and the polyaxial feature, could support new product development for orthopaedic, medical and industrial manufacturers. The technology is FDA-cleared and approved by Health Canada.
Source: Aurora Spine Corporation
Aurora Spine's proprietary implant locking technology, covered by U.S. Patent No. 9,603,637 (Polyaxial Interspinous Fusion Implant and Bone Growth Stimulation System), is available for out-licensing or private labeling opportunities.
Leadership notes that the device, with its combination of a ONE-STEP™ locking mechanism and the...
Aurora Spine’s proprietary implant locking technology, covered by U.S. Patent No. 9,603,637 (Polyaxial Interspinous Fusion Implant and Bone Growth Stimulation System), is available for out-licensing or private labeling opportunities.
Leadership notes that the device, with its combination of a ONE-STEP™ locking mechanism and the polyaxial feature, could support new product development for orthopaedic, medical and industrial manufacturers. The technology is FDA-cleared and approved by Health Canada.
Source: Aurora Spine Corporation

You’ve reached your limit.
We’re glad you’re finding value in our content — and we’d love for you to keep going.
Subscribe now for unlimited access to orthopedic business intelligence.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.