
 Copy to clipboard
Copy to clipboard 
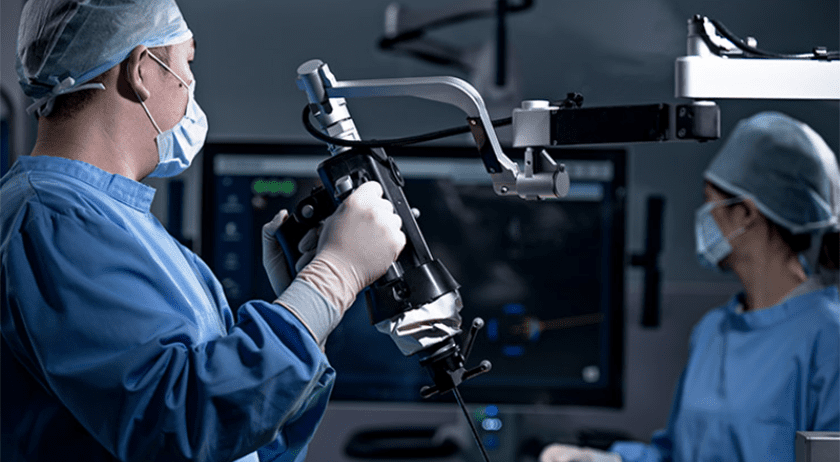
Point Robotics MedTech was granted FDA 510(k) clearance to market its minimally invasive surgical robot POINT™ Kinguide Robotic-Assisted Surgical System. This marks both Taiwan’s very first FDA-cleared surgical robot and what is reported to be the first ever hand-held robot framework equipped with a parallel manipulator for orthopedic application in the world.
The Kinguide Robotic-Assisted Surgical System helps the surgeon to drill bone and implant screws more efficiently and effectively. With a fully-integrated surgical system that includes both navigation and handheld drilling, the Kinguide system is designed to streamline procedural tasks and significantly reduce the surgeon’s burdens during implant surgery. Point Robotics next plans to expand the indication of Kinguide to other spinal surgery procedures, including herniated disc decompression.
“Precise positioning is the crucial factor for a successful spinal surgery,” said SC Juang, CEO of Point Robotics. “Wider application of orthopedic surgical robots in the spine, joints, and trauma surgeries will be the development trend in the coming decade. Following the clearance, Point Robotics is preparing for premarket submissions in Europe and China to jump start global deployments and to access international markets.”
Source: Point Robotics
Point Robotics MedTech was granted FDA 510(k) clearance to market its minimally invasive surgical robot POINT™ Kinguide Robotic-Assisted Surgical System. This marks both Taiwan’s very first FDA-cleared surgical robot and what is reported to be the first ever hand-held robot framework equipped with a parallel manipulator for orthopedic application...
Point Robotics MedTech was granted FDA 510(k) clearance to market its minimally invasive surgical robot POINT™ Kinguide Robotic-Assisted Surgical System. This marks both Taiwan’s very first FDA-cleared surgical robot and what is reported to be the first ever hand-held robot framework equipped with a parallel manipulator for orthopedic application in the world.
The Kinguide Robotic-Assisted Surgical System helps the surgeon to drill bone and implant screws more efficiently and effectively. With a fully-integrated surgical system that includes both navigation and handheld drilling, the Kinguide system is designed to streamline procedural tasks and significantly reduce the surgeon’s burdens during implant surgery. Point Robotics next plans to expand the indication of Kinguide to other spinal surgery procedures, including herniated disc decompression.
“Precise positioning is the crucial factor for a successful spinal surgery,” said SC Juang, CEO of Point Robotics. “Wider application of orthopedic surgical robots in the spine, joints, and trauma surgeries will be the development trend in the coming decade. Following the clearance, Point Robotics is preparing for premarket submissions in Europe and China to jump start global deployments and to access international markets.”
Source: Point Robotics

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







