
 Copy to clipboard
Copy to clipboard 
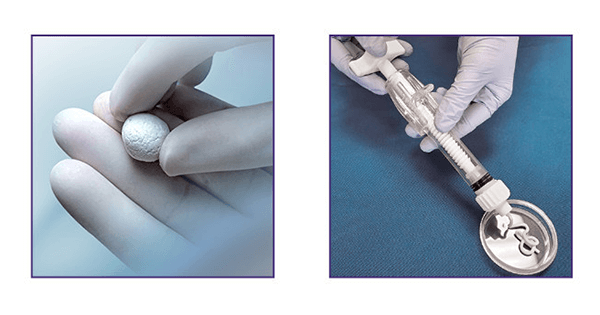
Paragon 28 launched MgNum™ Bone Void Filler for use in bony voids and defects that are not intrinsic to the stability of the bone structure.
Moldable and injectable MgNum BVF comprises magnesium, phosphates and a proprietary solution that hardens in situ at the defect site, and is receptive to drilling. MgNum BVF has a reported three times the compressive strength of cancellous bone, as well as adhesive properties.
Studies of MgNum BVF have demonstrated greater than 80% bone remodeling at 26 weeks. The product can be reconstituted with liquid, making it moldable for up to one hour.
“We are pleased to add MgNum BVF to our diverse biologics portfolio,” said Matt Jarboe, P28’s Chief Commercial Officer. “MgNum BVF allows us to more comprehensively support surgeons in indications we currently cover, and it will help us grow into new indications as well.”
Paragon 28 launched MgNum™ Bone Void Filler for use in bony voids and defects that are not intrinsic to the stability of the bone structure.
Moldable and injectable MgNum BVF comprises magnesium, phosphates and a proprietary solution that hardens in situ at the defect site, and is receptive to drilling. MgNum BVF has a reported three...
Paragon 28 launched MgNum™ Bone Void Filler for use in bony voids and defects that are not intrinsic to the stability of the bone structure.
Moldable and injectable MgNum BVF comprises magnesium, phosphates and a proprietary solution that hardens in situ at the defect site, and is receptive to drilling. MgNum BVF has a reported three times the compressive strength of cancellous bone, as well as adhesive properties.
Studies of MgNum BVF have demonstrated greater than 80% bone remodeling at 26 weeks. The product can be reconstituted with liquid, making it moldable for up to one hour.
“We are pleased to add MgNum BVF to our diverse biologics portfolio,” said Matt Jarboe, P28’s Chief Commercial Officer. “MgNum BVF allows us to more comprehensively support surgeons in indications we currently cover, and it will help us grow into new indications as well.”

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







